
The combination single CT scan for breast cancer staging and reconstruction
Introduction
Abdominal based flap reconstruction is considered by most to be the gold standard for breast reconstruction as it allows a large volume, autologous reconstruction. A systematic review by Tsoi has shown that it leads to a reduced risk of reconstructive failure as compared with prosthetic reconstruction (1). Since its description in 1994 by Allen and Treece (2) the deep inferior epigastric perforator (DIEP) flap is superior to the transverse rectus abdominal muscle (TRAM) flap, as it preserves abdominal wall musculature, leading to less post-operative pain, shorter post-operative stays and faster recovery (3). It also minimises donor site complications such as a bulge or hernia. Preoperative imaging has become routine in many centres around the world as it identifies perforators which are suitable for flap harvest (4). Computed tomographic angiography (CTA) has been shown to be superior to Doppler with intraoperative findings, reducing partial and total flap failure (5). This allows a preoperative decision to me made, as to which abdominal wall flap to use, which shortens the overall operating time. A common criticism is that CTA increases the radiation dose to the patient, due to the necessity of doing an extra scan. We have developed a technique of combining the CTA with the patient’s staging CT scan (chest, abdomen and pelvis) for breast cancer surveillance, thereby reducing both the number of scans and the subsequent radiation to the patient.
Methods
The CT scan parameters are summarized in Table 1. The first range is similar to Rozen’s CTA for DIEP protocol (5), in that the scan is in the arterial phase and performed in a caudal to cranial direction from the symphysis pubis. Our protocol differs in that instead of the scan finishing at 3 cm above the umbilicus, it is continued cranially to include the chest and finishes in the supraclavicular region. The second range is of the hepatic portal venous phase to identify hepatic metastases. This is performed in a cranial to caudal direction from the diaphragm to pubic symphysis. The third (equilibrium) phase looks for hypervascular tumours (HCC), blood pooling (haemangiomas) or scar tissue. This is delayed by 5 minutes and runs in a cranial to caudal direction again directly over the liver.
Table 1
| Scanner | Toshiba Aquilion One, helical multidetector |
|---|---|
| Slice thickness | 0.5×80 |
| Detector pitch | Standard PF 0.813, HP 65 |
| Gantry rotation speed | 0.6 sec (arterial) and 0.5 (portal venous and delayed) |
| Tube potential | 120 kV |
| Tube current | Smart mA: dose modulation varies with patient size and body region |
| IV contrast | Omnipaque 350 100 mLs |
| IV contrast rate | Single injector, 20 G Iv cannula, 4mLs/sec |
| Range 1 | Arterial phase—symphysis pubis to supraclavicular region |
| Range 2 | Portal venous phase—started 40 secs post arterial scan—diaphragm to pubic symphysis |
| Range 3 | Delayed liver at 5 minutes |
| Bolus tracking | No ROI used. Smart Prep sample at groin; “Go” when contrast seen in femoral artery. Manual trigger; scan delay is 4 secs from the press of the button |
DIEP, deep inferior epigastric perforator; PF, pitch factor; HP, helical pitch; CT, computed tomographic; ROI, region of interest.
Results
Since Jan 2012 we have used this CTA protocol technique in 68 consecutive patients with breast cancer, who have been referred to the senior author for breast reconstruction and were considered for immediate free DIEP or TRAM flap reconstruction. This has formed part of our previously published ‘reverse protocol’ (6) whereby patients who have locally advanced breast cancer undergo preoperative neoadjuvant chemotherapy then also receive radiotherapy before having a single stage mastectomy and autologous reconstruction. We have also used this CTA protocol successfully in patients who required staging prior to a mastectomy and reconstruction.
Of the total patients 66 underwent immediate DIEP reconstruction and 2 were done as delayed cases.
The scan quality achieved was universally excellent (Figure 1). The scan was then reformatted using the OsiriX programme (Figure 2) allowing preoperative planning of which perforators and type of flap to select. Intraoperative findings in both groups were consistent with the preoperative CTAs such that the intraoperative plan did not need to be changed compared with the preoperative findings. There were no complete or partial flap failures.
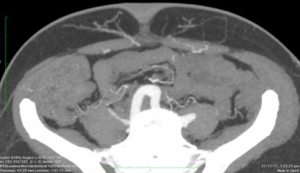
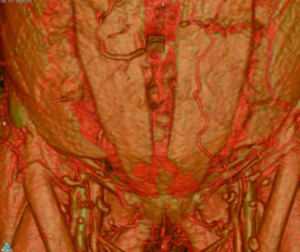
Discussion
Whilst CT scans are invaluable to the breast clinician for both staging and reconstructive purposes; there are unfortunately competing aims of each investigation and prior to this protocol it necessitated 2 separate scans (7). The main reason a staging CT scan could not be used for preoperative DIEP flap reformatting was due to the fact that the arterial phase of the scan was performed in a craniocaudal direction which is counterproductive to seeing the perforators of the deep inferior epigastric artery.
By reversing the direction of scanning in the arterial phase we can obtain the required information for DIEP planning whilst not affecting the oncologic staging all in the same scan. Radiology reporting of the staging scan has been unaffected by the change in protocol. Obviously, this is of great value to both the patient and the broader healthcare system in terms of time, resources and costs.
Another specific advantage of the “Combination Scan” is the reduction of the total irradiation to the individual, which may elevate a person’s lifetime risk of developing radiation induced cancer. To this end, there is a public health drive to reduce the amount of lifetime radiation that a patient is exposed to wherever possible (7).
The radiation dose range for this new staging-CTA is 8–14 mSv, which is the same as a staging chest, abdominal and pelvis CT. The range for a standalone CTA of the abdominal wall vasculature is 7–13 mSv—equivalent to 350–650 chest X-rays (7), which is therefore avoided with the new protocol. Whilst some authors suggest MRA as another option (8,9), this involves a further inconvenience to the patient, possibly delay in management and increased costs. Furthermore, there is a greater propensity for movement artefact with an MRA versus a CTA.
The “Combination Scan” has become the standard of care in our institution and has no downside. It has been successfully implemented with a DIEP flap survival of 100%.
Conclusions
We therefore propose this novel CT protocol for all patients who will be considered for breast cancer staging. It allows comprehensive staging in the appropriate patient and simultaneously allows the same images to be used for reformatting for DIEP flap reconstructions either as an immediate or delayed reconstruction.
Acknowledgments
The authors acknowledge Peter Barnes, National Radiation Safety Manager, I-MED Radiology Network, for assistance in the preparation of this manuscript.
Funding: None
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2018.03.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tsoi B, Ziolkowski N, Thoma A, et al. Safety of tissue expander/implant versus autologous abdominal tissue breast reconstruction in postmastectomy breast cancer patients: a systematic review and meta-analysis. Plast Reconstr Surg 2014;133:234-49. [Crossref] [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Butler PD, Wu LC. Abdominal perforator vs . muscle sparing flaps for breast reconstruction. Gland Surg 2015;4:212-21. [PubMed]
- Casares Santiago M, García-Tutor E, Rodríguez Caravaca G, et al. Optimising the preoperative planning of deep inferior epigastric perforator flaps for breast reconstruction. Eur Radiol 2014;24:2097-108. [Crossref] [PubMed]
- Rozen WM, Phillips TJ, Ashton MW, et al. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and Doppler ultrasound. Plast Reconstr Surg 2008;121:9-16. [PubMed]
- Grinsell D, Pitcher M, Wong S, et al. Immediate autologous breast reconstruction after neoadjuvant chemoradiotherapy for breast cancer: initial results of the first 29 patients. ANZ J Surg 2018;88:E137-41. [Crossref] [PubMed]
-
Initiative to Reduce Unnecessary Radiation Exposure from Medical Imaging - Cina A, Barone-Adesi L, Rinaldi P, et al. Planning deep inferior epigastric perforator flaps for breast reconstruction: a comparison between multidetector computed tomography and magnetic resonance angiography. Eur Radiol 2013;23:2333-43. [Crossref] [PubMed]
- Neil-Dwyer JG, Ludman CN, Schaverien M, et al. Magnetic resonance angiography in preoperative planning of deep inferior epigastric artery perforator flaps. J Plast Reconstr Aesthet Surg 2009;62:1661-5. [Crossref] [PubMed]
Cite this article as: Alexander KS, Baker C, Smith P, Grinsell D. The combination single CT scan for breast cancer staging and reconstruction. Ann Breast Surg 2018;2:9.

