
Breast sharing for closure contralateral mastectomy defect
Introduction
Oncoplastic technique is a valuable component of breast cancer surgery for patients who need to preserve their breast. Data also shows that it has oncological safety while maintaining the cosmetic result of the breast. In preserving the breast, resection of large tumors can be challenging. For locally recurrent breast cancer, the main goal of surgery is local disease control to palliate clinical symptoms. To make the oncoplastic surgery effective and oncologically safe, all foci of the cancers needs to be completely removed with adequate surgical margins giving enough histological normal tissue and maintaining the cosmetic result of the breast and there are no deformity sequelae.
Palliative systemic therapy is primarily used to treat breast cancer patients with concurrent distant metastases (stage IV). Surgical removal of the breast tumor does not provide survival benefit to the patients. Occasionally, primary tumors are resected in these patients for palliative reasons, such as improving the quality of life (QOL) through relieving symptoms of pain, infection, ulceration or bleeding. Without clear data for management recommendations, patients should be initiated on systemic therapy as first-line treatment. Patients who responded to treatments or had persistent, non-progressive metastatic diseases (especially those with good performance status) may be considered for palliative surgery as a salvage treatment. The QOL benefits have been highlighted in a recent study (1). A salvage resection was defined as the resection of all visible lesion, extended to the surrounding skin with a safety margin of at least 2 cm (2).
The choices of reconstruction methods depend on the location and size of the defect, availability of the local and pedicle flaps, previous surgery or radiotherapy, and the general condition of the patient. Direct simple closure is possible in small lesions. Skin grafts can usually be treated with superficial chest wall defects involving only the soft tissue. Previous or post-operative radiation therapy may compromise the healing of skin grafts.
Many kinds of flaps reconstruction were used to cover such chest wall defect, involving latissimus dorsi flap, transverse rectus abdominal myocutaneous flap, pectoralis major myocutaneous flap, and omental flap. However, these flaps may not be suitable for all patients regarding the existence of comorbidities and tissue availability (3). Thus, the local flap became one of the choices for these patients. In this study, we present one case that we used breast parenchyma to cover medial side chest wall defect in contralateral lesion.
Methods
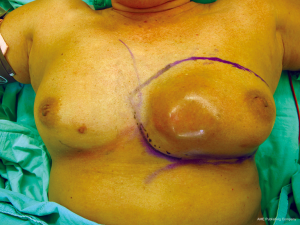
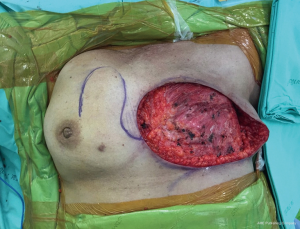
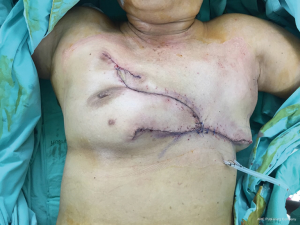
A 65-year-old patient presented with a large mass in her left breast. She was diagnosed with invasive ductal carcinoma. The assessment for metastatic disease showed no lesion of the chest and abdomen, but the bone scan found bone metastasis. She received systemic endocrine therapy for stage IV disease until stable bone metastases. After stable of bone disease was evaluated, salvage mastectomy was planned to reduce her symptoms in the breast. Preoperative breast lesion is shown in the picture (Figure 1). The lesion showed skin involvement mostly in the lower left of the inner breast and massive skin resection needs to be done. After total mastectomy was done, skin closure left was performed with direct closure between superior and inferior skin flap starting at the lateral site. However, it still left the defect in the left inner lower quadrant. We designed the breast flap from the upper part of right breast as a rotational flap to cover the medial defect of the left breast (Figure 2). After the breast flap was elevated from pectoralis major muscle, the incision was made along the previously drawn line. The base of the flap needs to be at least ½ wide in length so that its vascular supply can be maintained. The flap was then rotated clockwise into the defect and sutured to the remnant of skin. The defect was covered with a right breast flap (Figure 3). Two drains were put under the flap on the mastectomy bed. The patient was scheduled for the postoperative follow up at 1 week and the result is shown in Figure 4. The flap was totally viable with acceptable cosmetic result of the donor site.
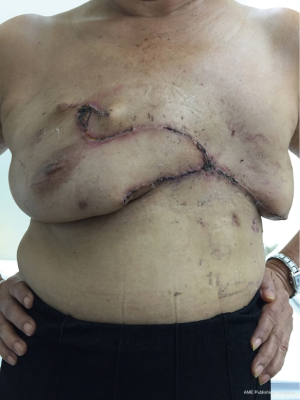
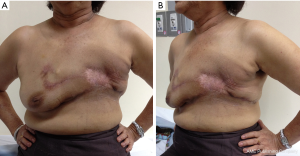
We discussed the role of chest wall radiation after surgery in our Breast Conference. Finally, the patient received chest wall radiation due to bone metastases improvement. The follow up of the flap post radiation are shown in Figure 5A and 5B.
Discussion
There are many choices for coverage of chest wall defects after mastectomy. Flaps are superior to skin graft in general, especially for those who need radiation afterwards, because of its aesthetic result and integrity. Pedicle TRAM flaps and latissimus dorsi flap are some examples of the myocutaneous flaps with reliable vascular supplies, which are suitable for covering the large chest wall defect. However, those flaps may not be suitable for all patients especially those with comorbidity. Moreover, local flaps enable surgeons to close large defects with a lower morbidity compared to the muscle flaps that are either pedicled or free. Many procedures share the common goal to provide good primary healing and reduce flap failure. Planning for these techniques, however, requires an adequate length-to-width ratio and knowledge of the vascular anatomy of the donor region.
Local flaps that have been reported in previous literatures are bilateral advancement flaps that gave a horizontal scar at the end. Some defects are too large in the vertical dimension, allowing the tension to eventually cause wound dehiscence (4). Other types of flaps such as thoracoabdominal flap are more suitable in case of large defect (less than 600 cm2) and better than myocutaneous flaps in terms of blood loss, operative time, and length of hospital stay (4,5). However, the major drawback of this procedure is the vertical midline scar (4). Thoracoepigastric flap is another option for large chest wall defect. Using mid axillary incision and base on perforators of superior-epigastric vessels, the flap was reported to be hemodynamically weak and behaves like random flap (4).
The breast parenchyma can be used as a flap to cover the defect located mainly in the midline. This flap is suitable for elderly patients with associated comorbidities because of its short operative time. The breast flap has excellent blood supply and is reliable, but it provides poor cosmetic outcome (6). In the literatures, breast flap had been used as a rotational flap to cover anterolateral chest wall defect as well (7). In addition, breast tissue was used for advancement flap to cover contralateral site defect as it was called ‘cyclops flap’. Its use was suitable in most patient but the cosmetic outcome was an issue since the nipple has to be in the middle of chest wall (8).
In our experience, we prefer the breast tissue as rotational flap for selected patients with medial side defect. As the blood supply for breast tissue comes from both side of the chest wall (mostly from internal mammary medially and lateral intercostal artery laterally), we selected the medial side flap above the nipple areolar complex and rotated in the defect. Since this local flap follows the random pattern of the internal mammary arcade and the subdermal plexus, the width-to-length of the flap doesn’t need to go beyond 1:2 (9). The breast flap contains full thickness of the skin. In this case, the breast flap was 100% viable and no necrosis of the flap was found postoperative. Even though slight deformity of the right breast was found, it was not major and the patient accepted the cosmetic result.
Conclusions
Due to a good blood supply of the breast parenchyma, breast flap is one of the interesting choices among the local flaps used for covering chest wall defect in contralateral mastectomy site. Breast flap gives a reliable vascular supply and no tissue necrosis was found. We concluded that this technique should be considered among patients with comorbidity or with limitation to other types of reconstructions.
Acknowledgments
The authors wish to acknowledge Miss Nichakarn Kuphirun for English revision of the text.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2018.06.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Veronesi G, Scanagatta P, Goldhirsch A, et al. Results of chest wall resection for recurrent or locally advanced breast malignancies. Breast 2007;16:297-302. [Crossref] [PubMed]
- Levy Faber D, Fadel E, Kolb F, et al. Outcome of full-thickness chest wall resection for isolated breast cancer recurrence. Eur J Cardiothorac Surg 2013;44:637-42. [Crossref] [PubMed]
- Aydin A, Güven E, Karaca B, et al. Reconstruction of the Chest Wall Defects with Mammary Dermoglandular Advancement Flaps in Female Complicated Cases. Trakya Univ Tip Fak Derg 2009;26:130-3.
- Park JS, Ahn SH, Son BH, et al. Using Local Flaps in a Chest Wall Reconstruction after Mastectomy for Locally Advanced Breast Cancer. Arch Plast Surg 2015;42:288. [Crossref] [PubMed]
- Deo SVS, Purkayastha J, Shukla NK, et al. Myocutaneous versus thoraco-abdominal flap cover for soft tissue defects following surgery for locally advanced and recurrent breast cancer. J Surg Oncol 2003;83:31-5. [Crossref] [PubMed]
- Tukiainen E. Chest wall reconstruction after oncological resections. Scand J Surg 2013;102:9-13. [Crossref] [PubMed]
- Taghizadeh R, Rampaul RS, O'Donoghue JM. Use of the ipsilateral breast in anterolateral chest wall reconstruction. J Plast Reconstr Aesthet Surg 2010;63:e779-81. [Crossref] [PubMed]
- Kent C. Hughes MJH, Jennifer Turner, Ernest K. Manders. Design of the Cyclops Flap for Chest-wall Reconstruction. Plast Reconstr Surg 1997;100:1146-51. [Crossref] [PubMed]
- Acea Nebril B, Builes Ramírez S, García Novoa A, et al. Rotational Flaps in Oncologic Breast Surgery. Anatomical and Technical Considerations. Cirugía Española 2016;94:372-8. (English Edition). [Crossref] [PubMed]
Cite this article as: Chirappapha P, Sukpanich R, Leesombatpaiboon M, Supsamutchai C, Sukarayothin T, Rattadilok C. Breast sharing for closure contralateral mastectomy defect. Ann Breast Surg 2018;2:11.

