
Novel algorithm in decision making process for implant-based breast reconstruction
Introduction
There has been a renewed interest in the pre-pectoral placement of tissue expanders and implants due to possibility of decreased animation deformity, pain, and hospital stays with similar aesthetic and complication profiles compared to sub-pectoral placement (1). This is supported by recent publications from experienced surgeons who often work with breast surgeons familiar with these procedures and whom are aware of the importance of healthy mastectomy flaps. However, for surgeons who are beginning to offer pre-pectoral device placement, or whom work with breast surgeons who are unfamiliar with this approach, pre-pectoral reconstruction can be daunting.
The benefits of a direct-to-implant (DTI) reconstruction have also recently become more touted due to its appeal of being a single stage operation with a comparable complication profile to a two-stage approach. However, the loss of a DTI reconstruction can be devastating and subsequently requires at least two additional procedures and/or an autologous reconstruction.
The senior author has developed a step-wise algorithm from the initial consultation to the operating room in an attempt to optimize patient satisfaction, aesthetic outcomes, and minimize complications for pre-pectoral and DTI device placement. We provide technical pearls, potential pitfalls, and results for each pathway.
Pre-operative consultation
When the patient presents for a pre-operative consultation, the surgeon must become familiar with the patient’s preference and understanding of her reconstructive options, which can be based on pre-conceived notions from social media, self-education or discussion with other patients. In general, the ability to reconstruct the breast is limited by the demand placed on the mastectomy flaps due to either inherent (patient-related) or iatrogenic (surgery-related) factors. Thus, the ability to determine the appropriate avenue of breast reconstruction must be based on patient factors such a co-morbidities, smoking history and prior surgeries, the expected oncologic course, such as need for radiation therapy, as well as expected intra-operative factors related to flap perfusion.
Pre-pectoral reconstruction can be a powerful tool in the appropriately selected patient. However, patient selection remains critical. Generally speaking, patients who are deemed to be potential pre-pectoral candidates should be offered both pre- and sub-pectoral reconstruction with the understanding that the final decision will be made intra-operatively. Similarly, patients with the above relative contraindications can be offered a pre-pectoral placement with an emphasis that it will most likely be with a tissue expander as opposed to implant and that they are at higher risk of potential complications including implant failure. Table 1 lists absolute and relative pre-operative contraindications to pre-pectoral placement.
Table 1
| Patient/surgical factors |
| Active or recent smoking history |
| Obesity (BMI ≥35) |
| Poor mastectomy flap perfusion |
| Neo-adjuvant radiation/previous radiation to the chest wall |
| Prior sub-pectoral implant augmentation |
| Poorly controlled diabetes |
| Oncologic factors |
| Aggressive axillary metastasis and/or bulky adenopathy |
| Stage IV breast cancer |
| Inflammatory breast cancer |
| Proximity (≤0.5 cm) to the pectoralis major muscle |
| Chest wall tumor invasion |
Once a surgeon determines that a patient is eligible for pre-pectoral reconstruction, the decision of tissue expander placement as opposed to immediate implant placement (DTI) must be made. The relative contraindications to a DTI approach are identical to those of pre-pectoral placement. In women who desire to be larger breasted, consideration should be given to placement of a tissue expander. If a woman is undergoing unilateral reconstruction, a tissue expander is typically utilized as there is a high likelihood she will undergo a matching procedure on the contralateral side. However, if a patient opts for a single-stage reconstruction, the contralateral matching procedure can be done at the same time as the mastectomy. Finally, women who may undergo or known to need adjuvant radiation should be considered for expander placement as well to allow for modification of the volume based on treatments and tissue changes. Regardless of reconstructive decision, time must be spent with the patient discussing expectations and the likelihood of achieving those goals with a single as opposed to staged operation.
Intra-operative
The reconstructive plan should be communicated to the breast surgeon to ensure attention is paid to mastectomy flap thickness, especially when a pre-pectoral reconstruction is being considered.
Intra-operatively, the decision on which type of reconstruction to pursue should be made based on the viability of the mastectomy flaps, the tension on closure and the ultimate desired size, in a step-wise fashion.
Mastectomy flap viability can be evaluated in a multitude of ways, but should be assessed in all cases (3-6). Evaluation can be made clinically (capillary refill, skin edge bleeding, exposed dermis) or with the assistance of technological adjuncts, such as utilizing Indocyanine dye. If the viability of the mastectomy flaps is sub-optimal, a sub-muscular tissue expander should be placed as to not further compromise the vascularity of the flaps which would lead to wound healing complications or skin flap necrosis. If the flaps appear optimal, the tension on closure should then be assessed.
The tension upon closure is important; however, its importance is influenced by flap viability. A moderate amount of tension can be tolerated in robust mastectomy flaps while a small amount of tension in compromised flaps can potentially result in significant compromise. If there is tension on closure, a pre-pectoral tissue expander should be placed, as this will allow for easy volume adjustment should there be a vascular embarrassment post-operatively. If the tension is optimal, the ultimate desired size of the patient should be taken into account.
While ultimate breast size can be discussed with the patient pre-operatively, this also must be considered intra-operatively once mastectomy flaps and residual tissue can be examined. If a pre-pectoral implant can provide the desired size, it can be placed. However, if delivering the desired size would place undue tension on the closure, a pre-pectoral tissue expander should be placed. Patients who desire a small size can benefit from a concurrent or delayed skin reduction procedure. Also, if the patient was unsure of what final size she desired, she may benefit from an initial tissue expander placement to allow for size adjustments. A graphic representation can be found in Figure 1.
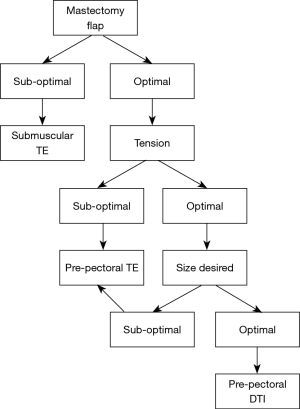
The second stage with the pre-pectoral tissue expander to implant exchange is demonstrated in Figure 2.
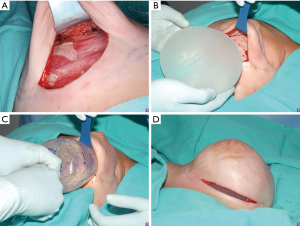
Changing pocket to pre-pectoral placement
A similar algorithm can be followed when conversions from a sub-pectoral plane to a pre-pectoral plane. Indications for this procedure include animation deformity, poor aesthetic outcome, pain, capsular contracture and patient preference (2). In this algorithm, patients that were previously not pre-pectoral reconstruction candidates may become candidates following a first stage sub-muscular reconstruction. In these instances, a similar algorithm can be followed, but less importance is placed on the viability of the mastectomy flaps as time for healing has occurred. However, for patients who have had radiation therapy, the viability of the flaps is not as predictable and must be carefully assessed.
In some instances, when converting to a pre-pectoral plane, it may be wise to utilize a tissue expander in a two-stage approach. This approach should be considered for patients who have been previously radiated, smokers, or had mastectomy flaps of questionable viability. Similarly, patients desiring to change their size, especially requesting a larger size would benefit from tissue expander placement.
Case examples
Case #1 pre-pectoral tissue expander reconstruction
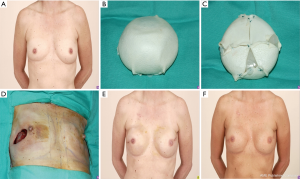
A 53-year-old female with left breast DCIS underwent a two-stage reconstruction with tissue expander placement at time of mastectomy. The tissue expander was inflated to the desired size and then wrapped circumferentially with AlloDerm. This patient underwent one round of fat grafting and a right nipple reduction to achieve desired post-operative results (Figure 3).
Case #2 pocket exchange from sub-muscular to pre-pectoral plane
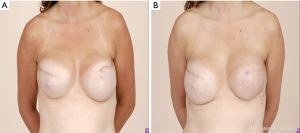
A 42-year-old female who underwent a prior nipple-sparing mastectomy at an outside hospital presented to our office with grade 4 capsular contracture on the right. She subsequent underwent a bilateral capsulectomy with transition to pre-pectoral pocket (Figure 4).
Discussion
There has been a recent surge in the interest and utilization of pre-pectoral breast reconstruction for a variety for reasons. As patients become more aware of and educated on this technique, there is likely to be a commensurate rise in patient requests for this procedure. In this paper, we have presented a novel algorithm for determining which patients are pre-pectoral candidates and we have identified patient factors that are amenable to a DTI approach as opposed to a two-stage reconstruction with a tissue expander. Finally, we discussed considerations for patients undergoing pocket conversion from a sub-pectoral to a pre-pectoral plane. The pre-pectoral technique is an exciting avenue with numerous potential patient benefits. However, reconstruction failure remains a devastating complication and this proposed algorithm allows physicians to maximize their results while minimizing potential complications.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2018.08.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). All patients provided written consent to proceed with the procedure.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nahabedian MY, Cocilovo C. Two-Stage Prosthetic Breast Reconstruction: A Comparison Between Prepectoral and Partial Subpectoral Techniques. Plast Reconstr Surg 2017;140:22S-30S. [Crossref] [PubMed]
- Sbitany H. Important Considerations for Performing Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:7S-13S. [Crossref] [PubMed]
- Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg 2014;101:899-911. [Crossref] [PubMed]
- Sbitany H, Wang F, Peled AW, et al. Tissue Expander Reconstruction After Total Skin-Sparing Mastectomy: Defining the Effects of Coverage Technique on Nipple/Areola Preservation. Ann Plast Surg 2016;77:17-24. [Crossref] [PubMed]
- Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 2012;129:778e-88e. [Crossref] [PubMed]
- Mattison GL, Lewis PG, Gupta SC, et al. SPY Imaging Use in Postmastectomy Breast Reconstruction Patients: Preventative or Overly Conservative? Plast Reconstr Surg 2016;138:15e-21e. [Crossref] [PubMed]
Cite this article as: Gatherwright J, Knackstedt R, Djohan R. Novel algorithm in decision making process for implant-based breast reconstruction. Ann Breast Surg 2018;2:15.

