Investigation of the relationship between the cranial collector and the incidence of lymphedema in patients with breast cancer after adjuvant treatments
Introduction
Upper extremity lymphedema (LE) is a well-known complication and a cause of severe morbidity in patients with breast cancer undergoing axillary lymph node dissection (ALND) (1). ALND is associated with a 14–40% risk of LE, and even sentinel lymph node biopsy (SNB) is associated with a 6–10% risk (1-3). First described by Thompson et al. and Nos et al. in 2007, axillary reverse mapping (ARM) procedures may reduce the incidence of LE (4,5). ARM was developed to identify and preserve the lymphatic ducts of the arm during ALND or SNB, thereby minimizing the incidence of LE. Tummel et al. reported that ARM was effective for reducing of the incidence of LE with oncologic safety, which were low rates of metastases in identified lymph nodes derived from the extremity (ARM nodes) and low axillary recurrence rate in the patients who underwent ARM procedures with a SLNB and/or ALND (6). However, a comprehensive review of ARM in breast cancer surgery revealed that 0–52% of successfully identified ARM nodes harbored cancer (7-9). We also reported breast cancer metastasis in ARM nodes in 24% and 2.9% of clinically node-positive and node-negative axilla patients, respectively (10).
Thus, there is a need to identify other ways to reduce the incidence of LE by using information from the ARM rather than sparing the ARM nodes or lymphatic ducts in the ALND field. Pavlist and colleagues reported that the individual lymphatic drainage collectors from the upper extremities to the axilla in cadavers were divided into a cranial, a caudal and a medial collector according to the location and connection with axillary lymph nodes (11). The cranial collector (CC) mostly traveled along the axillary vein and was a direct connection between the upper extremities and the apex of the axilla. In contrast, the caudal and medial collectors led to the central area of the axilla and often connected the central axillary lymph nodes. The authors speculated that preservation of any these collectors during ALND might reduce the risk of LE. We also previously reported that a CC running near the axillary vein was a negative risk factor for LE (12). However, patients with breast cancer who undergo ALND often require several adjuvant treatments after surgery. These adjuvant treatments, especially chemotherapy and radiotherapy, could influence the residual lymphatic drainage pathways of postoperative patients, which may lead to LE (13-22). The present study focused on the potential use of the CC to avoid the development of LE and investigated the differences in the incidence of LE according to the CC status and adjuvant treatments in patients who underwent ALND.
Methods
Patients and breast cancer treatments
From December 2009 to April 2018, 1,352 patients with primary breast cancer underwent breast surgery at our hospital. Of these patients, 339 required ALND and 101 were enrolled in the first our previous clinical study (10,12), and 116 were enrolled in the second our clinical study. The period of the first study was from December 2009 to November 2012 and the second study underwent from July 2014 to April 2018. Both studies were approved by the institutional review board of our hospital. Written informed consent was obtained from all of the patients who participated in the studies. In this study, we retrospectively investigated the data of the patients with jointing our two previous studies. Of 217 enrolled patients combing ALND, six were lost to follow-up and three had missing data regarding their lymphatic pathway during operation. Finally, we retrospectively evaluated the relationship between the CC status and LE incidence after adjuvant treatments, such as radiation therapy and chemotherapy, in 208 patients (Table 1). Their mean age and body mass index (BMI) were 58.5 (range, 33–88) years and 23.4 (range, 13.5–53.4) kg/m2, respectively. Primary systemic therapy (PST) was administrated to 51 (24%) of the 208 patients: the regimens were most commonly anthracycline and/or taxane-based chemotherapy. Among fifty-one clinically node-positive patients who received PST, 17 (33%) patients had a conversion to pathologically node negative status in consequence of ALND. Adjuvant chemotherapy was administered to 95 (46%) of the 208 patients: the regimens were also anthracycline and/or taxane-based chemotherapy in 82 (86%) of the 95 patients. Postoperative radiation was administered to 90 (43%) of the 208 patients: all patients who received postoperative mastectomy radiation therapy (PMRT) underwent both chest wall and supraclavicular radiation therapy (SCRT), whereas 12 (24%) of 50 patients who received whole breast radiation had undergone SCRT.
Table 1
| Characteristics | Value (n=208) |
|---|---|
| Surgery, n [%] | |
| Lumpectomy | 58 [28] |
| Mastectomy | 137 [66] |
| ALND alone | 13 [6] |
| PST, n [%] | |
| A.-based CT | 47 [23] |
| T.-based CT | 45 [22] |
| Others | 5 [2] |
| None | 157 [76] |
| Adjuvant treatments, n [%] | |
| A-based CT only | 21 [10] |
| T-based CT only | 7 [3] |
| A and T CT | 54 [26] |
| Other CT | 10 [5] |
| Tamoxifen | 63 [30] |
| Aromatase inhibitor | 75 [36] |
| Trastuzumab | 32 [15] |
| None | 22 [11] |
| Radiation therapy, n [%] | |
| WBRT | 38 [18] |
| WBRT + SCRT | 12 [6] |
| PMRT | 0 [0] |
| PMRT + SCRT | 40 [19] |
| None | 118 [57] |
| Clinical stage, n [%] | |
| I | 33 [16] |
| II | 109 [52] |
| III | 54 [26] |
| IV | 2 [1] |
| Unknown | 10 [5] |
| Subtype, n [%] | |
| Luminal | 129 [62] |
| Luminal—Her-2 | 31 [15] |
| Her-2 en-riched | 17 [8] |
| Triple negative | 24 [12] |
| Unknown | 7 [3] |
| The mean number of LNs [range] | |
| Removed LNs | 14.5 [0–34] |
| Positive LNs | 3.3 [0–26] |
ALND, axillary lymph node dissection; PST, primary systemic treatment; A, anthracycline; T, taxane; CT, chemotherapy; WBRT, whole breast radiation therapy; SCRT, supraclavicular radiation therapy; PMRT, postmastectomy radiation therapy; LNs, lymph nodes.
SNB procedure
In the patients with clinically node-negative axilla, 1–2 mL of indigocarmine (Daiichi Sankyo, Co., Ltd., Tokyo, Japan) was administered via intra-dermal injection to the areolar region with a 26-gauge needle after the induction of general anesthesia and before the patient was prepared for surgery. ALND was performed if frozen sections of the sentinel nodes revealed micrometastasis.
ARM and ALND procedure
Patients with clinically node-positive axilla initially received an intra-dermal injection of 0.5–1.0 mL (5 mg/mL) indocyanine green as a tracing agent (ICG; Diagnogreen; Daiichi Sankyo, Co., Ltd., Tokyo, Japan) at the upper and inner ipsilateral arm using a 1-mL syringe with a 26-gauge needle under general anesthesia before preparation for breast surgery. After massaging for approximately 5 min, the fluorescence signal was detected using an invisible near-infrared fluorescence imaging system (PDETM; Photo Dynamic Eye; Hamamatsu Photonics Company, Hamamatsu, Japan.) as flowing to the axilla from the upper extremity. Patients who had positive sentinel nodes as shown on SLNB received an ICG injection before ALND in a manner similar to that for patients with clinically node-positive axilla. After performing lumpectomy or mastectomy for breast cancer, we started to explore ARM nodes in the axillary field. ARM nodes and lymphatic ducts were identified as fluorescent objects in the axilla even if they did not exhibit a green-colored stain. The locations of all identified ARM nodes were marked on the map according to the surgical landmarks of the axilla as our previously reported. The limits of ALND were defined by the axillary vein, superiorly; the anterior serratus muscle, medially; and the anterior edge of the latissimus dorsi muscle, laterally. When fluorescent ARM nodes were located in the ALND field, they were separately removed to evaluate the pathological findings. On the other hand, when identified ARM nodes were outside the ALND field, they were spared. When we would start to dissect the just below the axillary vein, we evaluated the existence of CC not to disrupt the CC running around the vein during lymph node dissection.
Evaluation of LE
A systematic review and meta-analysis of lymphedema after breast cancer revealed that the most common method of lymphedema measurement was arm circumference and the incidence of arm lymphedema seemed to increase with time up to 2 years from diagnosis or surgery, after which incidence seemed to decrease (23). So, LE was assessed by circumferential arm measurements collected at baseline (admission date), 6 months postoperatively, and annually for two or three years postoperatively. We measured both arms at four locations; i.e., the palm, wrist, 5 cm below the elbow, and 10 cm above the elbow, as described by the Japanese Breast Cancer Society (24). A 2-cm increase at any level relative to the baseline or the healthy opposite arm was defined as LE positivity.
Evaluation of the LE incidence-CC status, and adjuvant treatments
We investigated the effect of chemotherapy or radiotherapy, especially anthracycline and/or taxane-based chemotherapy or supraclavicular radiation, on the incidence of LE according to the CC status.
Statistical analysis
Stat-Mate version IV for Windows (ATMS Company, Tokyo, Japan) was used to perform statistical analyses. We used the chi-squared test or the Mann-Whitney U tests to estimate the differences in the clinico-pathological and ARM-associated data of the LE-positive and LE-negative patients. Two-tailed P values <0.05 were considered statistically significant. The odds ratios for LE incidence with respect to the multivariate clinicopathological and ARM-associated data were calculated using a logistic multivariate regression model. The Kaplan-Meier method was used to estimate and plot the cumulative incidence of LE with or without radiation therapy and adjuvant chemotherapy.
Results
Incidence of LE
At a median follow-up of 24.2 months (range, 0–39 months), 68 of 208 (32.7%) patients had developed LE. LE first occurred within 6 months after surgery in 34%, one year in 66%, 2 years in 86%, and 3 years in 96% of these patients. Eight patients developed LE a few days after surgery: none of these patients had a CC in the ALND field. The patients with LE received manual lymphatic drainage from medical lymphatic drainage therapists as soon as possible at the division of outpatient service for LE in our institute.
Identification of CC and ARM nodes in node positive patients
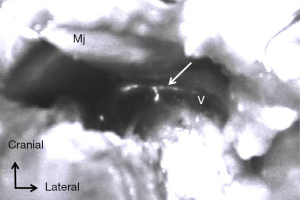
Under fluorescence imaging using a PDETM System, we identified ARM lymph nodes and CC in 185 (89%) patients and 81 (39%) patients, respectively (Figure 1). All identified CC could be preserved, whereas the identified ARM nodes could be spared in 12 of 185 patients because these nodes were mostly located in ALND field at the axilla. Metastatic ARM nodes were identified in 68 of 177 (38.4%) patients with ARM nodes removed from the ALND field.
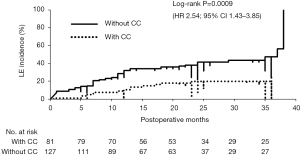
Relationship between the CC and adjuvant treatment on the incidence of LE
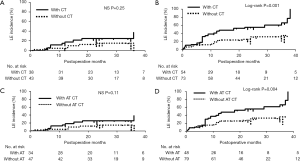
We investigated how adjuvant treatments, radiotherapy, or chemotherapy affected the incidence of LE in patients with or without CC (CC status). CC-negative patients had a significantly greater incidence of LE compared to that in CC-positive patients [log-rank tests; P=0.00069: HR: 2.54; 95% confidence interval (CI), 1.43–3.85] (Figure 2). Among 127 CC-negative patients, younger age, greater number of metastatic lymph nodes, and adjuvant chemotherapy were significantly positive risk factors for the incidence of LE (P=0.002, 0.05, and 0.002, respectively). In contrast, positive ARM lymph nodes and radiation therapy were significant risk factors for LE in 81 CC-positive patients (P=0.047 and 0.0009, respectively) (Table 2). In univariate analysis, anthracycline and/or taxane-based chemotherapy was significantly used in the patients with LE compared to the patients without LE in CC-negative patient group (chi-square test; P=0.02). Conversely, SCRT was significantly performed for the patients with LE compared to the patients without LE in CC-positive patient group (chi-square test; P=0.0008) (Table 3). The estimate of the cumulative incidence of LE with the Kaplan-Meier method indicated that anthracycline and/or taxane-based chemotherapy was a significant positive risk factor for LE in CC-negative patients (log-rank tests; P=0.004; HR: 2.1; 95% CI, 1.31–4.28) (Figure 3). And SCRT was a significant risk factor for LE in CC-positive patients (log-rank tests; P=0.0001; HR: 5.76; 95% CI, 3.29–41.2) (Figure 4).
Table 2
| Variables | CC positive patients (n=81) | CC negative patients (n=127) | |||||
|---|---|---|---|---|---|---|---|
| LE+ [n=15] | LE− [n=66] | P value | LE+ [n=53] | LE− [n=74] | P value | ||
| Age | 54.6±12.9 | 59.2±12.1 | 0.30 | 54.5±11.2 | 61.3±13.5 | 0.002 | |
| BMI | 22.9±4.1 | 22.9±4.5 | 0.84 | 24.1±4.1 | 23.5±5.3 | 0.18 | |
| Positive LNs | 0.76 | 0.05 | |||||
| 0–3 | 12 [80] | 55 [83] | 34 [64] | 59 [80] | |||
| ≥4 | 3 [20] | 11 [17] | 19 [36] | 15 [20] | |||
| Removed LNs | 13.9±6.1 | 13.2±5.6 | 0.85 | 15.9±7.0 | 14.7±7.2 | 0.38 | |
| Positive ARM LNs | 0.047 | 0.40 | |||||
| Yes | 8 [53] | 15 [23] | 21[40] | 24 [32] | |||
| No | 4 [27] | 37 [56] | 26 [49] | 42 [57] | |||
| Unknown | 3 [20] | 14 [21] | 6 [11] | 8 [11] | |||
| PST | 0.09 | 0.98 | |||||
| Yes | 6 [40] | 13 [20] | 13 [25] | 18 [24] | |||
| No | 9 [60] | 53 [70] | 40 [75] | 56 [76] | |||
| Adjuvant chemotherapy | 0.10 | 0.002 | |||||
| Yes | 11 [73] | 30 [45] | 31 [58] | 23 [31] | |||
| No | 4 [27] | 36 [55] | 22 [42] | 51 [69] | |||
| Radiation therapy | 0.0009 | 0.819 | |||||
| Yes | 12 [80] | 22 [33] | 24 [45] | 32 [43] | |||
| No | 3 [20] | 44 [67] | 29 [55] | 42 [57] | |||
Data are shown as mean ± SD or number [percentage]. LE, lymphedema of the upper extremity; CC, cranial collector; BMI, body mass index (kg/m2); LNs, lymph nodes; ARM LNs, identified lymph nodes derived from the upper extremity by axillary reverse mapping; PST, primary systemic treatment.
Table 3
| Variables | CC positive patients (n=81) | CC negative patients (n=127) | |||||
|---|---|---|---|---|---|---|---|
| LE+ (n=15) | LE− (n=66) | P value | LE+ (n=53) | LE− (n=74) | P value | ||
| Adjuvant chemotherapy, n [%] | |||||||
| A and/or T CT | 0.20 | 0.02 | |||||
| Yes | 9 [60] | 25 [38] | 25 [47] | 20 [27] | |||
| No | 6 [40] | 41 [62] | 28 [53] | 54 [73] | |||
| Radiation therapy, n [%] | |||||||
| SCRT | 0.0008 | 0.98 | |||||
| Yes | 9 [60] | 12 [18] | 13 [25] | 18 [24] | |||
| No | 6 [40] | 54 [82] | 40 [75] | 56 [76] | |||
LE, lymphedema of the upper extremity; CC, cranial collector; A, anthracycline; T, taxane; CT, chemotherapy; SCRT, supraclavicular radiation therapy.
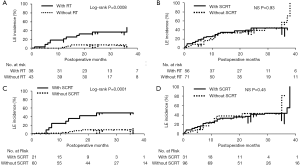
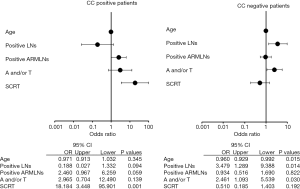
Multivariate analysis of LE risk factors revealed younger age, greater number of positive lymph nodes, and anthracycline and/or taxane-based chemotherapy to be independent risk factors for LE in CC-negative patients, while SCRT was an independent risk factor in CC-positive patients (Figure 5).
Discussion
A number of studies have reported the risk factors of breast cancer-related LE, including ALND, postoperative regional node radiation, chemotherapy, higher BMI, and older age (1-4,13-23,25-28). In addition, we reported that the lack of CC and RM node metastasis based on ARM could be independent risk factors for LE in patients undergoing ALND (12). Although the existence of the CC is a negative risk factor for LE, patients with a preserved CC during ALND may have a further risk of developing LE due to adjuvant treatments. In this investigation, postoperative radiation, especially SCRT, was associated with incidence of LE in CC-positive patients. In contrast, adjuvant chemotherapy, especially anthracycline and/or taxane-based chemotherapy, was associated with the incidence of LE in CC-negative patients. Previous studies have also indicated that adjuvant chemotherapy is a potential risk factor for LE (13,17). Taxane-based chemotherapy is of interest in breast cancer-related LE literature because of taxane-induced fluid retention in patients during treatment (13,14,17-21). Nguyen et al. reported in a large cohort study that anthracycline chemotherapy (without RT) increased the 5-year incidence of breast cancer-related lymphedema (BCRL) from 3.5% (ALND only) to 8.4%, a different that was not statistically significant (P=0.10), while anthracycline plus taxane chemotherapy had a much higher 5-year incidence of 33.6% (P<0.001) (22). We could not distinguish which anthracycline or taxane would impact the incidence of LE in CC-negative patients because both anthracycline and taxane were used sequentially in most patients in the present study. However, previous studies reported taxane-based chemotherapy maybe a risk factor of LE (19-22,25). In the present study, PST was not a risk factor for LE. Taxane-based chemotherapy was a risk factor for LE in the adjuvant but not the neo-adjuvant setting. Park et al. reported that the change in BMI was most significant just after the fourth cycle of taxane in the neo-adjuvant setting; however, the change in body weight was not a significant factor for the incidence of LE (26). Kim et al. also reported that adjuvant chemotherapy was not significantly correlated with LE in 313 patients with clinically node-positive breast cancer who underwent neoadjuvant chemotherapy followed by surgery with ALND (27). The systemic edema caused by taxane in adjuvant setting may lead to LE due to the disruption of the lymphatic pathways in the axilla by ALND. This assumption may be supported by the observation that anthracycline and/or taxane-based chemotherapy was associated with a higher incidence of LE in CC-negative patients only. The CC might have a role in preventing the development of LE by addressing systemic edema related to the side effects of taxane. Especially, docetaxel causes systemic edema more frequent than paclitaxel according to the review (25). As one of the possibilities how the CC status would be the clinical consequences and implications, we would choice paclitaxel as adjuvant chemotherapy instead of docetaxel for CC-negative patients. Although the identification rate of CC was 39% that is relatively low, we expect that it could be useful to reduce the incidence of LE by changing adjuvant treatments according to the CC status in future.
A number of studies reported radiation therapy to be a major and independent risk factor for LE (13,15-18,28). Warren et al. demonstrated that regional lymph node radiation, including SCRT, significantly increased the risk of LE compared to breast or chest wall radiation alone (HR: 1.7; 95% CI: 1.07–2.7) (28). In this study, SCRT was a risk factor for LE in CC-positive patients. We hypothesize that SCRT may result in the development of fibrosis surrounding the tissue where the CC was running, which may interrupt the lymphatic flow from the CC. Because the CC generally travels along the axillary vein, the SCRT field may be planned to include this area. Planning of the SCRT to avoid the CC pathway in CC-positive patients may reduce the incidence of LE in this group of patients. This supposition should be assessed in a clinical trial in addition to oncologic safety for local recurrence because the radiation filed would be partially omitted. In contrast, CC-negative patients may require more intensive care when taxane-based chemotherapy is administered. Thus, CC status might be also useful when planning surveys for LE after ALND.
Surgeons may not perform ALND in clinically-negative axilla patients with either negative or one or two positive sentinel nodes by SNB (29-33). Several studies have assessed the feasibility of omitting ALND in patients with clinically-positive axilla, who converted to clinically-negative axilla following neo-adjuvant chemotherapy (34,35). Furthermore, ongoing prospective randomized phase III clinical trials are evaluating the role of ALND or regional nodal radiation therapy in patients with breast cancer with positive sentinel lymph node disease after neoadjuvant chemotherapy (36,37). However, the patients who do not meet the criteria for inclusion in these clinical trials are still at risk of LE after ALND. Information from the ARM may be helpful in reducing the risk of LE or planning surveys for LE in patients requiring ALND.
To our knowledge, this is the first report to evaluate the impact of postoperative radiation (especially, SCRT) or adjuvant chemotherapy (anthracycline and/or taxane-based chemotherapy) on the incidence of LE according to ARM lymphatic drainage pathways. However, the limitations of our current study are retrospective investigation and including the patients who received various treatments in addition to ALND. Further studies are needed to investigate how to apply this information to reduce the incidence of LE in patients who require ALND.
Conclusions
In our series, the CC status of patients undergoing ALND impacted on the incidence of LE after chemotherapy and radiation therapy.
Acknowledgments
The authors thank the surgical and other co-medical staffs in our institute for their assistance with this study.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2019.01.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was carried out with jointing our two previous studies based on the approval by the institutional review board of our hospital (approval number 695 in the first study and 1406040 in the second study). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McLaughlin SA. Lymphedema. In: Harris JR, Lippman ME, Morrow M, et al. editors. Diseases of the Breast. Fifth edition. Alphen aan den Rijn: Wolters Kluwer Health Adis (ESP), 2014:590-601.
- Stamatakos M, Stefanaki C, Kontzoglou K. Lymphedema and breast cancer: a review of the literature. Breast Cancer 2011;18:174-80. [Crossref] [PubMed]
- Rockson SG. Lymphedema after breast cancer treatment. N Engl J Med 2018;379:1937-44. [Crossref] [PubMed]
- Thompson M, Korourian S, Henry-Tillman R, et al. Axillary reverse mapping (ARM): A new concept to identify and enhance lymphatic preservation. Ann Surg Oncol 2007;14:1890-5. [Crossref] [PubMed]
- Nos C, Leiseur B, Clough KB, et al. Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary dissection. Ann Surg Oncol 2007;14:2490-6. [Crossref] [PubMed]
- Tummel E, Ochoa D, Korourian S, et al. Does Axillary Reverse Mapping Prevent Lymphedema After Lymphadenectomy? Ann Surg 2017;265:987-92. [Crossref] [PubMed]
- Shao X, Sun B, Shen Y. Axillary reverse mapping (ARM): where to go. Breast Cancer 2019;26:1-10. [Crossref] [PubMed]
- Seyednejad N, Kuusk U, Wiseman SM. Axillary reverse lymphatic mapping in breast cancer surgery: A comprehensive review. Expert Rev Anticancer Ther 2014;14:771-81. [Crossref] [PubMed]
- Han C, Yang B, Zuo WS, et al. The Feasibility and Oncological Safety of Axillary Reverse Mapping in Patients with Breast Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS One 2016;11:e0150285 [Crossref] [PubMed]
- Ikeda K, Ogawa Y, Komatsu H, et al. Evaluation of the metastatic status of lymph nodes identified using axillary reverse mapping in breast cancer patients. World J Surg Oncol 2012;10:233-9. [Crossref] [PubMed]
- Pavlista D, Eliska O. Relationship between the lymphatic drainage of the breast and the upper extremity: A postmortem study. Ann Surg Oncol 2012;19:3410-5. [Crossref] [PubMed]
- Ikeda K, Ogawa Y, Kajino C, et al. The influence of axillary reverse mapping related factors on lymphedema in breast cancer patients. Eur J Surg Oncol 2014;40:818-23. [Crossref] [PubMed]
- Gillespie TC, Sayegh HE, Brunelle CK, et al. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surg 2018;7:379-403. [Crossref] [PubMed]
- Swaroop MN, Ferguson CM, Horick NK, et al. Impact of adjuvant taxane-based chemotherapy on development of breast cancer-related lymphedema: results from a large prospective cohort. Breast Cancer Res Treat 2015;151:393-403. [PubMed]
- Sayegh HE, Asdourian MS, Swaroop MN, et al. Diagnostic methods, risk factors, prevention, and management of breast cancer-related lymphedema: past, present, and future directions. Curr Breast Cancer Rep 2017;9:111-21. [Crossref] [PubMed]
- Shaitelman SF, Chaiang YJ, Griffin KD, et al. Radiation therapy targets and the risk of breast cancer-related lymphedema: a systemic review and network meta-analysis. Breast Cancer Res Treat 2017;162:201-15. [Crossref] [PubMed]
- Zou L, Liu FH, Hu Y, et al. The incidence and risk factors of related lymphedema for breast cancer survivor post-operation: a 2-year follow-up prospective cohort study. Breast Cancer 2018;25:309-14. [Crossref] [PubMed]
- Kim M, Kim SW, Lee NK, et al. A model to estimate the risk of breast cancer-related lymphedema: combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys 2013;86:498-503. [Crossref] [PubMed]
- Cariati M, Bains SK, Grootendorst MR, et al. Adjuvant taxanes and development of breast cancer-related arm lymphedema. Br J Surg 2015;102:1071-8. [Crossref] [PubMed]
- Zhu W, Li D, Li X, et al. Association between adjuvant docetaxel-based chemotherapy and breast cancer-related lymphedema. Anticancer Drugs 2017;28:350-5. [Crossref] [PubMed]
- Hugenholtz-Wamsteker W, Robbeson C, Nijs J, et al. The effect of docetaxel on developing oedema in patients with breast cancer: a systemic review. Eur J Cancer Care (Engl) 2016;25:269-79. [Crossref] [PubMed]
- Nguyen TT, Hoskin TL, Habermann EB, et al. Breast cancer related lymphedema risk is related to multidisciplinary treatment and not surgery alone – results from a large cohort study. Ann Surg Oncol 2017;24:2972-80. [Crossref] [PubMed]
- DiSipio T, Ryo S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systemic review and meta-analysis. Lancet Oncol 2013;14:500-15. [Crossref] [PubMed]
- Kitamura K, Akazawa K. Multi-center survey of breast cancer related arm lymphedema and future issues. J Jpn Coll Angiol 2010;50:715-20.
- Qin YY, Li H, Guo XJ, et al. Adjuvant chemotherapy, with or without taxanes, in early or operable breast cancer: A meta-analysis of 19 randomised trials with 30698 patients. PLoS One 2011;6:e26946 [Crossref] [PubMed]
- Park S, Lee JE, Yu J, et al. Risk factors affecting breast cancer-related lymphedema: serial body weight change during neoadjuvant anthracycline plus cyclophosphamide followed by taxane. Clin Breast Cancer 2018;18:e49-54. [Crossref] [PubMed]
- Kim M, Park IH, Lee KS, et al. Breast cancer-related lymphedema after neoadjuvant chemotherapy. Cancer Res Treat 2015;47:416-23. [Crossref] [PubMed]
- Warren LE, Miller CL, Horick N, et al. The impact of radiation on the risk of lymphedema after treatment for breast cancer: A prospective cohort study. Int J Radiat Oncol Biol Phys 2014;88:565-71. [Crossref] [PubMed]
- Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011(Alliance) Randomized Clinical Trial. JAMA 2017;318:918-26. [Crossref] [PubMed]
- Donker M, Tienhoven GV, Meijnen P, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomized, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. [Crossref] [PubMed]
- Lyman GH, Somerfield MR, Bosserman LD, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol 2017;35:561-4. [Crossref] [PubMed]
- Işık A, Grassi A, Soran A. Positive axilla in breast cancer; clinical practice in 2018. Eur J Breast Health 2018;14:134-5. [PubMed]
- McCartan D, Gemignani ML. Current management o the axilla. Clin Obstet Gynecol 2016;59:743-55. [Crossref] [PubMed]
- Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph nodes surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the American college of surgeons oncology group (ACOSOG) Z1071 clinical trial. JAMA 2013;310:1455-61. [Crossref] [PubMed]
- Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013;14:609-18. [Crossref] [PubMed]
- NCI Community Oncology Research Program - Protocol Summary. Alliance A011202 - A Randomized Phase III Trial Evaluating the Role of Axillary Lymph Node Dissection in Breast Cancer Patients (CT1-3 N1) Who Have Positive Sentinel Lymph Node Disease After Neoadjuvant Chemotherapy. Available online: https://www.kccop.org/cancer-trials/breast/index.cgi/summary?tID=110 [cited 2018 27 Nov].
- NCI Community Oncology Research Program - Protocol Summary. NSABP B-51 – A Randomized Phase III Clinical Trial Evaluating Post-Mastectomy Chestwall and Regional Nodal XRT and Post-Lumpectomy Regional Nodal XRT in Patients with Positive Axillary Nodes Before Neoadjuvant Chemotherapy Who Convert to Pathologically Negative Axillary Nodes After Neoadjuvant Chemotherapy. Available online: https://www.kccop.org/cancer-trials/breast/index.cgi/summary?tID=92 [cited 2018 27 Nov].
Cite this article as: Ikeda K, Ogawa Y, Kamei Y, Watanabe C, Tokunaga S, Fukushima H, Inoue T. Investigation of the relationship between the cranial collector and the incidence of lymphedema in patients with breast cancer after adjuvant treatments. Ann Breast Surg 2019;3:4.