An extended 7-year review of textured breast implants for primary breast augmentation: Allergan® versus Mentor®
Introduction
Breast augmentation remains one of the most commonly performed aesthetic procedures around the world. The British Association of Aesthetic Plastic Surgeons (BAAPS) publishes annual statistics from the United Kingdom, with over 8,200 breast augmentations performed in 2017, which was a 6% increase from the previous year (1).
Textured implants first emerged in the 1970s in the form of second-generation polyurethane-textured implants with the aim of reducing the rate of capsular contracture. In 1992, Silicone implants were taken off the market by the FDA in the United States (US) amidst safety concerns. In 2006, Mentor® and Allergan® were given approval from the FDA to market fourth generation silicone implants in the US (2).
There has been a continued dichotomy in the type of breast implants used by surgeons in the US; favouring smooth silicone implants, and the rest of the world; which favour textured silicone gel implants. Textured implants have been a popular choice due to lower rates of capsular contracture, the predictability of placement and increased pocket control (3-5).
Over the previous decades there have been several public ‘scares’ relating to breast implants. This includes the highly publicised PIP implant scandal and more recently the rising concern surrounding breast implant associated anaplastic large cell lymphoma (BIA-ALCL).
To date, BIA-ALCL has only been detected in patients that have had previous exposure to textured implants. No case of BIA-ALCL has arisen in patients with solely smooth implants. Up until this month the guidance from governing bodies advised against any change in implant practice. Despite this, a national survey in the UK published earlier this year showed up to a third of surgeons had already made a change to their implant practice following rising concerns of BIA-ALCL in textured implants (6).
On the 16th of December 2018, the CE mark for Microcell® and Biocell® implants manufactured by Allergan® expired. The Medicines and Healthcare products Regulatory Agency (MHRA) has advised all remaining implants and expanders are to be withdrawn throughout Europe. The British Association of Plastic, Reconstructive and Aesthetic Surgeons (BAPRAS) president David Ward released a statement regarding discontinuing these Allergan implants the same week.
We performed a 7-year retrospective review of bilateral breast augmentations (BBA) using a majority of Allergan® implants. Given this recent withdrawal from the market we felt it was a pertinent time to release our long-term follow up data and complication rates.
Methods
Aims
The aim of this study was to determine the complications and outcomes of primary BBA performed by the senior author. This includes a comparison of outcomes between two implant manufacturers used in our practice (Allergan® and Mentor®). In addition, we wanted to correlate these outcomes with the rising concerns surrounding BIA-ALCL development in patients with textured implants, specifically Allergan textured implants.
Data collection
Data was collected on all primary BBA that were performed between the 1st of January 2010 to the 31st of December 2016 by the senior author. Patient demographics collected included age, body-mass index, pre-operative breast cup size, past medical history and the use of anticoagulants.
Peri-operative information included; Pre-operative breast shape, size and ptosis. Surgical technique used included; Incision, pocket type, implant washing and specifics regarding the implant used (make, size, shape, texturing, projection, height).
Post-operative data was collected up until discharge and any consultation or communication regarding complications up until the patient charts were reviewed in October 2018 were included.
End-points of interest are sub-divided into early and late complications. Early complications include; haematoma, seroma, infection, wound dehiscence, nipple sensation, pathological scarring and return to theatre. Late complications include; late seroma, implant rupture, capsular contracture, rippling, residual asymmetry or the development of BIA-ALCL.
We excluded any patients who had adjunctive procedures, e.g., augmentation mastopexy or those who had a unilateral augmentation as part of a symmetrising procedure. We also excluded any secondary cases, e.g., exchange of implants, capsule procedures and reconstruction cases.
Surgical technique
The senior author who performed all of the procedures in this series has maintained a consistent approach to BBA throughout. Preoperatively, a single dose of prophylactic intravenous antibiotic is administered by the anaesthetist at induction. All patients are prepped with betadine and sterile drapes are used ensuring the axilla is covered. The nipples are not routinely covered.
All BBAs were performed through an infra-mammary incision with dissection using a ceramic-tipped epitome to create an implant pocket either in the subglandular plane or the dual plane technique under direct vision. We do not routinely wash out the pocket nor use introducer sleeves. Before placement of the implant, the surgeon, scrub nurse nor the surgical assistant change their gloves.
Textured implants from two manufacturers were used over the 7-year period; Mentor® and Allergan®. Each implant is opened immediately before insertion and a new bottle of Videne (Povidone-Iodine 10% w/w cutaneous solution; Ecolab, Minnesota) is opened and poured into the plastic container to surround the implant. A small amount of this Videne is also put around the infra-mammary incision prior to implant insertion. Closure is performed in three layers using 3-0 Monocryl. Dressings used include Steristrips (3M, Minneapolis), dressing gauze and a Tegaderm barrier dressings (3M, Minneapolis). No drains were used in this series.
Postoperatively, patients wore a pre-fitted non-wired sports bra and routinely were kept overnight for observation. They were discharged the following day and appointed to return to the outpatient clinic for consultant review in six weeks.
Results
Overall, 172 primary BBA were performed in this time period. The average patient age was 31 years. The average BMI was 22. All patients were healthy at baseline with no significant medical comorbidities. None of the patients were taking any anticoagulants or antiplatelet medications (Table 1 and Figure 1).
Table 1
| Patient and surgical data | Total patient numbers | Percentage % |
|---|---|---|
| Patient demographics | ||
| Mean age (years, range) | 31 (range, 15–51) | |
| BMI (mean, kg/m2) | 22 (range, 18–33) | |
| Anticoagulant use (n, %) | 0 | |
| Pre-op breast assessment | ||
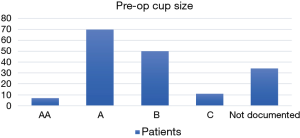
| Cup size | AA: 7 | AA: 7/138=5% |
| A: 70 | A: 51% | |
| B: 50 | B: 36% | |
| C: 11 | C: 8% | |
| Not documented: 34 | ||
| Ptosis grade (I–III) | I: 62 | I: 62/136=46% |
| II: 42 | II: 31% | |
| III: 32 | III: 23% | |
| Not documented: 36 | ND | |
| Other | Asymmetry: 34 | Asymmetry: 20% |
| Chest wall deformity: 8 | Chest wall deformity: 5% | |
| Tuberous breasts: 6 | Tuberous breasts: 3% | |
| Implant/technique | ||
| Implant manufacturers (n, %) | Allergan | 103 (60%) |
| Mentor | 69 (40%) | |
| Mean implant volume (mL, range) | 318 cc | Mode 300 cc (165–415 cc) |
| Implant shape (n, %) | Round | 162 (94%) |
| Anatomical | 10 (6%) | |
| Textured vs. smooth | Textured | 172 (100%) |
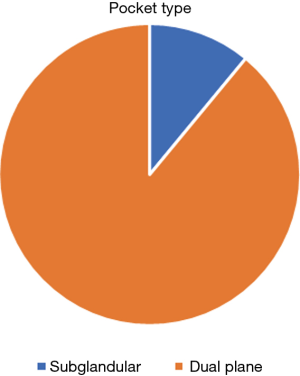
| Implant pocket | Dual plane | 153 (89%) |
| Subglandular | 19 (11%) |
All implants inserted were textured and via an incision in the infra-mammary fold. The majority of implants were Allergan® (60%) and the remaining were Mentor® (40%). The majority (94%) were round implants and 89% were inserted into a dual plane pocket with only 11% being placed in a subglandular pocket (Figure 2). The commonest implant size inserted was 300 cc, the average implant volume was 318 cc with the range from 165 to 415 cc (Table 1).
Early/mid-complications (Table 2)
Table 2
| Early complications | Overall | Allergan | Mentor |
|---|---|---|---|
| Total no. | 172 | 103 | 69 |
| Haematoma | 2 (1%) | 2 (2%) | 0 |
| Seroma | Early =1; late =2 | 3 (3%) | 0 |
| Infection | 0 | 0 | 0 |
| Wound dehiscence | 0 | 0 | 0 |
| Scarring (hypertrophic) | 1 | 1 (1%) | 0 |
| Altered nipple sensation | 1 | 0 | 1 (1%) |
| Late complications | |||
| Rippling | 2 | 2 (2%) | 0 |
| Ptosis | 3 | 1 (1%) | 2 (3%) |
| Capsule formation (grade 2–4); overall 2% | Grade 2 =2; grade 3 =1; grade 4 =0 | 3 (3%) | 0 |
| Implant leakage/rupture | 6 ruptures (3%) | 5 (5%) | 1 (1%) |
| BIA-ALCL | 0 | 0 | 0 |
BIA-ALCL, breast implant associated anaplastic large cell lymphoma.
Two patients in the study developed a post-operative haematoma (1%). Both of these patients required a return to theatre for evacuation of the haematoma and washout of the cavity within 24 hours. Neither of these patients went on to develop any other late complications, specifically capsular contracture and they have remained well throughout the follow-up period.
One patient developed an early seroma within 6 weeks which resolved spontaneously. We had no patients with post-operative wound infections or wound dehiscence. One patient reported nipple hypersensitivity and another developed a hypertrophic scar.
The average active follow-up time was 16.9 weeks and the passive follow-up time was 352.1 weeks (6.8 calendar years). Active follow up included the post-operative review period prior to discharge. Passive follow up was the time from the operation to the chart review in October 2018. This captured any patients who returned at a later stage with mid- and late-term complications. Four plastic surgeons work in this particular cosmetic practice, which is one of only four cosmetic practices in Northern Ireland. If any patients consulted one of the other plastic surgeons regarding complications in the passive follow up period, this was also included in the data.
Three patients consulted the senior surgeon either by email or in person regarding residual Ptosis. Two of these patients proceeded to have a mastopexy following their original bilateral augmentation. One of these patients had originally been advised she required an augmentation mastopexy but declined it at the time as she did not want the extra scars associated with the mastopexy. All three of these patients were documented pre-operatively as having a grade 3 Ptosis.
Overall there were 6 implant ruptures (3%). Five of these were Allergan® implants (83%) and one Mentor® implant (17%). Only one of these patients reported any preceding trauma to explain the rupture, the remainder were therefore classed as atraumatic. The average time between the original implant insertion and the replacement of the ruptured implant was 5 years (range, 2–8 years).
Three patients developed a documented capsule of Baker grade II or above. All 3 were in patients with Allergan® implants. Two patients that developed capsules had their implants placed in the dual plane and one was in a subglandular pocket. Only one patient had a grade III capsule and this occurred following an implant rupture.
Two patients (1%) had a late seroma documented. One was minimal and resolved spontaneously. The second patient had both cytology and a biopsy which were tested for BIA-ALCL markers and both specimens were negative. Over the extended 7-year follow up period to 2018 none of the patients in this series had a confirmed diagnosis of BIA-ALCL.
Discussion
Mentor® and McGhans’ (later known as Allergan®) fourth generation silicone gel implants were first approved by the FDA in the US in November 2006, 14 years after silicone implants were removed from the market due to safety concerns. This approval followed preliminary 3-year data from a prospective clinical trial known as the Core trial which was led by Allergan®. The three main concerning complications surrounding breast implants are capsular contracture, implant rupture and now, one of the biggest concerns is the development of BIA-ALCL (2).
The core trial published their 10-year results in 2014. The trial involved Allergan® Natrelle round silicone gel implants, 56% of implants inserted were smooth and 44% were the Biocell® textured version which were used in our study. In keeping with our cohort, the commonest size of implant placed was 300 cc. Their study included revision augmentations and reconstruction augmentations with only 64% (455/715) being primary breast augmentations as was the case in our study. In addition, they performed adjunct procedures in 15% of primary augmentation cases and over 90% of reconstruction cases, both of these were excluded from our study (2).
Overall, our complication rates were lower than those published in the official Core trial. In Particular, the risk of rupture in our study was 5% in Allergan® implants compared to a rate of 9% in the Core study. The rate of capsular contracture was 3% for Allergan® implants in our study but in the augmentation only group in the Core Trial the rate published was significantly higher at 20%. The Core study also stated there was no significant difference in capsular contracture rate between textured and smooth implants (17% vs. 20%) which is an interesting finding (2).
Another paper by Doren et al. which was part of the FDA clinical trials for the fourth-generation silicone implants compared the outcomes between Allergan®, Mentor® and Sientra®. These were specifically contoured implants as opposed to round. In keeping with the Core study, the cohort of patients included primary augmentation, revision augmentation and reconstruction cases. Primary augmentation was performed in 384 patients (55%). Overall they showed the group who had Mentor® implants inserted for a primary augmentation had the lowest complication rate (7).
The majority of patients in our study had round implants (94%) rather than anatomical or contoured which makes it difficult to compare to the paper by Doren et al. Another paper published as part of the prospective FDA trial by Cunningham and McCue looks specifically at Mentor® round memory gel implants and is more applicable for comparison with our results. They had 558 women who had a primary augmentation. Their published rupture rate was 1% for the primary augmentation cohort which was the same as our 1% rupture rate in the mentor group. Capsular contracture rates (Baker Grade III/IV) however were higher at almost 9% compared to 0% in our cohort, it must be noted that our Mentor® group only had 69 patients in it compared to 558 in the trial but the follow up time was similar (8).
Overall in our cohort 3% of patients developed a Baker capsule of grade II or above. Out of the three patients only one (1%) had a grade III capsule which is what many other trials use as the cut off for the diagnosis of capsular contracture. Several theories and contributing factors to capsule formation have been well published. The commonest theory involves a biofilm surrounding the implant following bacterial contamination or infection (9).
A paper published by Chong and Diva recommend 14 steps to take to reduce the risk of capsular contracture. Amongst these 14 steps they recommend IV antibiotics at induction, a preference towards a dual-plane pocket and avoidance of the peri-areolar incision to reduce bacterial contamination. All of our implants are placed via the infra-mammary fold and all patients receive a dose of intravenous antibiotics at induction, 89% had a dual-plane pocket with our capsule rates being split 2:1 for dual plane Vs subglandular. Several of the remaining 14 points cover good surgical technique and haemostasis which should be the standard of any plastic surgeon (9).
Of the remaining 14 steps, there are four key steps they recommend that the senior author of this paper does not routinely do. These include the use of nipple covers, a sleeve for implant insertion, irrigation of the pocket with either antibiotics or iodine and changing both drapes and gloves before handling the implants. Despite this, we have a very low rate of capsular contracture in our series when compared to the trials discussed above. It is noteworthy that both implant biofilm and bacterial contamination are not only attributed to the development of capsular contracture but they have also been implicated in the development of BIA-ALCL (9).
BIA-ALCL
BIA-ALCL is a newly emerging neoplasia associated with breast implants, and in particular textured implants. As of March 2018, there were over 520 confirmed cases worldwide according to the American Society of Plastic Surgeons (ASPS) website. The world leading expert on BIA-ALCL, Mark Clemens quoted an up to date figure of 656 cases diagnosed worldwide at the BAPRAS Winter meeting in London, November 2018 (6,10).
The risk of BIA-ALCL development varies depending on the implant type and there also appears to be a geographical implication. The ASPS quote a risk between 1:3,817 and 1: 30,000. A risk of 1: 24,000 has been reported in the UK from a BAPRAS press release which followed the Panorama program ‘The Great Implant Scandal’ which aired in November 2018. A paper published by Loch-Wilkinson et al. from Australia and New Zealand in 2017 gave an implant—specific risk which appeared to correlate with higher textured implants. They compared the risk of developing BIA-ALCL to Siltex® implants as a baseline. Worryingly they quoted a 14-fold increased risk with Biocell® textured implants and an 11-fold increase with polyurethane textured implants (6,10,11).
A recent publication by Magnusson et al. has updated this information following the diagnosis of 26 additional cases of BIA-ALCL. They have re-calculated the implant specific risk as 23 times higher with polyurethane textured implants in comparison to Siltex®, with Biocell® risk increasing marginally to 16-fold. They conclude this is further evidence of the causal role of textured implants in this disease process, specifically in surface type 3 and 4 implants. They also recommend implant terminology should be changed from nominal terms; ‘micro’ and ‘nano’, to numerical, which better represents the level of texturing and therefore the associated risk level of developing BIA-ALCL (12).
The presentation, investigation and treatment of patients with suspected or confirmed BIA-ALCL is beyond the scope of this paper but it is covered in more detail in a paper we published earlier this year which we would direct you to (6).
On the 16th of December 2018, the CE mark expired on all textured Allergan® Natrelle series implants due to safety concerns. According to the MHRA it will not be renewed and all current stocks of these implants and tissue expanders throughout Europe are to be recalled. The CE mark represents approval that a product meets the European Union (EU) safety, health or environmental requirements, it is an indicator of compliance with EU legislation and allows free movement of products within the EU.
This happened shortly after the French regulatory authority, the Agence Nationale de Sécurité du Médicament (ANSM) recommended the use of smooth over textured implants amidst associations of textured implants with BIA-ALCL (13).
Prior to this landmark withdrawal of one of the commonest used implants in the UK and Europe, the governing bodies in the UK had not recommended any change in implant practice with the current level of evidence. A national survey throughout the UK and Ireland in 2018 found a shifting trend to surgeons offering patients the choice of either a smooth or textured implant after informed counselling on the risk: benefit ratio. At this stage up to a third of surgeons surveyed had either already changed their implant practice to smooth, nano-, or micro-textured implants or had plans to do so in the near future despite the advice from governing bodies at that time (6).
Informed patient consent remains a key priority and all patients should be given the most up to date information on the risk of BIA-ALCL and the particular types of implants it is associated with. Surgeons need to ensure they are up to date with the current literature which is evolving at a rapid pace. With the modern-day influence of social media and the internet, patients will have some knowledge of the current implant scandal from the medias perspective and it is our duty as caregivers to ensure they have accurate and up to date information. Indeed, it is not only a moral duty but in the United Kingdom it is a legal obligation following the change in consent laws after the Montgomery ruling in 2015 (14). We counsel all our patients on the implant specific risks and discuss the recent removal of several implants from the market. The senior author has now changed to using smooth implants in all primary breast augmentations.
Throughout this extended 7-year review period we had no patients diagnosed with BIA-ALCL. This included both Allergan® and Mentor® implants and all of the implants used in the study were textured. The average lead time from implant insertion to diagnosis of BIA-ALCL is 8–10 years but cases have been published with a lead time of less than one year before diagnosis. Certainly, these figures are confirmed in the most recent paper published by Magnusson et al. this year, they quote an average onset of 7.48 years but the range started from 6 months post-implant insertion to 25 years later. Although our follow up time does not extend to 10 years yet, we thought it was still worth including that currently none of our patients have developed BIA-ALCL to date. We are aware that this may change in future and the development of BIA-ALCL still remains a possibility, even up to 25 years later, as shown by Magnusson et al. (12). In addition, given the most recent update that the MHRA have withdrawn the same Allergan® implants from the European market for this reason we felt it was an appropriate time to publish our implant specific outcomes. Interestingly, in the Core Trial, only 5 patients were diagnosed with a late seroma and there were no cases of BIA-ALCL in the cohort of 715 patients.
Conclusions
Our review has shown excellent outcomes of 172 patients following primary breast augmentation. We have shown a low rate of capsular contracture and implant rupture and no cases of BIA-ALCL in our cohort to date. These outcomes are either similar or better than the results published of the 10-year outcomes of Natrelle implants in the official Allergan® trial and in the equivalent Mentor® trial.
Worrying however is the safety concern that remains over the link between BIA-ALCL and Allergan Biocell® implants which has led to the withdrawal of the CE mark and recall of these implants throughout Europe. Certainly, figures published in New Zealand gave a 16-fold increase risk of developing BIA-ALCL with Biocell® textured implants compared to another manufactured brand of textured implants (Siltex®) (11,12).
Despite these concerns, neither our cohort of 172 patients nor the Core trial of 715 patients had any cases of BIA-ALCL diagnosed. It must be mentioned however that if the risk of BIA-ALCL development quoted for Biocell® implants is 1:3,345 then it is possible that across a total of 887 patients none of them developed BIA-ALCL. The lag time between implant insertion and development (8–10 years) must also be considered as mentioned above and it is possible that some of these patients will go on to develop BIA-ALCL in the future.
These recent landmark developments lead us to wonder what the future holds, not only for Allergan® implants but for all textured implants on the market worldwide due to the rising cases of BIA-ALCL throughout the world.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2019.06.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). No ethical approval was required for this follow up study and informed consent was not required as there is no patient identifiable information or photographs used.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- British Association of Aesthetic Plastic Surgeons (BAAPS). Cosmetic surgery stats: Dad bods and filter jobs. Last updated: 27 Mar 2019 13:41. Available online: https://baaps.org.uk/about/news/1535/cosmetic_surgery_dad_bods_and_filter_jobs
- Spear SL, Murphy DK, Allergan Silicone Breast Implant US.Core Clinical Study Group. Natrelle round silicone breast implants: Core Study results at 10 years. Plast Reconstr Surg 2014;133:1354-61. [Crossref] [PubMed]
- Coleman DJ, Foo IT, Sharpe DT. Textured or smooth implants for breast augmentation? A prospective controlled trial. Br J Plast Surg 1991;44:444-8. [Crossref] [PubMed]
- Malata CM, Feldberg L, Coleman DJ, Foo IT, Sharpe DT. Textured or smooth implants for breast augmentation? Three year follow-up of a prospective randomised controlled trial. Br J Plast Surg 1997;50:99-105. [Crossref] [PubMed]
- Calobrace MB, Schwartz MR, Zeidler KR, et al. Long-Term Safety of Textured and Smooth Breast Implants. Aesthet Surg J 2017;38:38-48. [Crossref] [PubMed]
- Martin S, McBride M, Khan K. The current UK perspective of breast surgeons on breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Eur J Plast Surg 2019;42:43-8. [Crossref]
- Doren EL, Pierpont YN, Shivers SC, et al. Comparison of Allergan, Mentor, and Sientra Contoured Cohesive Gel Breast Implants: A Single Surgeon's 10-Year Experience. Plast Reconstr Surg 2015;136:957-66. [Crossref] [PubMed]
- Cunningham B, McCue J. Safety and effectiveness of Mentor's MemoryGel implants at 6 years. Aesthetic Plast Surg 2009;33:440-4. [Crossref] [PubMed]
- Chong SJ, Deva AK. Understanding the Etiology and Prevention of Capsular Contracture: Translating Science into Practice. Clin Plast Surg 2015;42:427-36. [Crossref] [PubMed]
- American Society of Plastic Surgeons Website. Accessed December 2018. Available online: https://www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-physician-resources/frequently-asked-questions
- Loch-Wilkinson A, Beath KJ, Knight RJW, et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk. Plast Reconstr Surg 2017;140:645-54. [Crossref] [PubMed]
- Magnusson M, Beath K, Cooter R, et al. The Epidemiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand Confirms the Highest Risk for Grade 4 Surface Breast Implants. Plast Reconstr Surg 2019;143:1285-92. [Crossref] [PubMed]
- International Consortium of Investigative Journalists. Accessed December 2018. Available online: https://www.icij.org/investigations/implant-files/allergan-textured-breast-implants-recalled-in-europe-pending-safety-review/
- Mercer NS. Perspective from the United Kingdom on Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J 2019;1:ojz003
Cite this article as: Martin SV, Ho W, Khan K. An extended 7-year review of textured breast implants for primary breast augmentation: Allergan® versus Mentor®. Ann Breast Surg 2019;3:14.