Pathological complete response in the axillary lymph nodes post neo-adjuvant chemotherapy in breast cancer, is it predictable?
Introduction
Breast cancer surgery has evolved over the years specially with the advances in the medical oncology therapies. The fast-growing field of the neo-adjuvant chemotherapy (NACT), with the ongoing introduction of new medications including targeted chemotherapy and immunotherapy has changed the management approach to specific subtypes of breast cancer. The National Surgical Adjuvant Breast and Bowel Project NSABP B-18 trial (1) has clearly demonstrated the significant benefit of NACT, not only in downsizing the breast tumour, but also in down staging the disease in node positive patients on the breast and the axillary response.
Magnetic resonance imaging (MRI) is being used in many centres including ours as a tool to assess the response of the breast tumour to NACT. Although controversial, there is still no clear-cut evidence to support MRI reliability in confirming the type and extent of the response (2,3). Nevertheless, it is one of the main tools to assist the surgeon’s decision in downsizing the breast tumour and allow breast conserving surgery (BCS). This unfortunately has not been replicated in practice in conserving the axilla when complete radiological axillary response is being observed on different imaging modalities; MRI or ultrasound scan (USS) of the axilla. The extensive potentially morbid axillary node clearance (ANC) is still the gold standard in such patients in many practices. Unfortunately, many of these axillary clearances show pCR on the final histology which means that this group of patients has been exposed to an unnecessary potentially morbid procedure. The number of this subgroup of patients is evidently increasing with the recent introduction of new medical treatments as alluded to above.
In the early days of breast cancer surgery, ANC was a routine axillary procedure regardless of its involvement. Complications included lymphedema (2–28%), shoulder function impairment (5–19%), dysesthesia and pain (23–31%) observed in some studies (4). With the trend of downsizing the breast cancer surgical management, axillary node sampling (ANS) was initially introduced in clinically negative axilla which eventually was replaced by sentinel lymph node biopsy (SLNB) which is currently being the gold standard axillary staging procedure with possible axillary clearance in positive SLNB (5-8). However, more recently SLNB has become the definitive procedure in a subset of patients where there is low metastatic burden in the axilla (1–2 positive SLNBs) in tumours ≤5 cm as clearly demonstrated in the controversial Z11. In this trial these patients had BCS with whole breast radiotherapy plus appropriate adjuvant systemic therapy, there 5-year survival was similar to those who underwent ANC for a similar group of patients (9,10). Some centres would offer axillary RT in case of positive axilla, instead of ANC in selected patients, this was tested by the AMAROS trial which studied whether axillary RT could yield comparable outcomes to ANC with fewer adverse side effects (11). The investigators reported similar distant-metastasis free survival and overall survival; however axillary recurrence was higher in the RT group (1.82% compared to 0.93% in ANC group) at 10-year follow-up. Not only the AMAROS trial was underpowered, but also the radiation field and technique used in the trial are different for today’s practice, which may influence the results either way.
With the success achieved by downsizing the breast surgery after NACT to an extent where BCS has been used in the good responders to confirm a pCR rather than a true oncological resection of the area in question. This concept has urged many to achieve a parallel result in the surgical management of the known metastatic axilla by performing a re-staging procedure after NACT instead of ANC, this would certainly help to do less surgery in the axilla and potentially avoid the morbid consequence of an excessive axillary surgery. The SENTINA (12) was one of the very first trials to test this hypothesis, its primary endpoint was accuracy (false-negative rate FNR) of SLNB after NACT for patients who converted from clinically node positive (cN1) to ycN0 disease during NACT. They reported a high FNR of 14.2% that can be lowered to 7.3% by removing at least 3 SLNBs post NACT.
Similarly, the (ACOSOG) Z1071 trial (13) showed an FNR of 12.6% in SLNB post NACT in initially cN1 patients, this was improved by clipping the node that was biopsied initially, and then targeting the clipped node when restaging the axilla.
More recently, Caudle et al. (10) has evaluated the effect of the NACT on the clipped node when performing the targeted axillary dissection (TAD) as part of the planed ANC, to determine if the changes seen in the clipped node would reflect the response of the whole axillary LNs, they found 2% FNR in TAD followed by ANC, and concluded “Marking nodes with biopsy-confirmed metastatic disease allows for selective removal and improves pathologic evaluation for residual nodal disease after chemotherapy”. More studies are ongoing for further evaluation of re-staging the axilla following the NACT by performing the TAD procedure to determine its role in reducing the unnecessary axillary dissection while ensuring optimum local disease control.
While most of these studies are trying to find the ideal axillary re-staging technique with the lowest FNR, a question rises to whether re-staging should be offered to all post NACT patients or to a selected group of patients with a predictable pCR in the axilla?
And hence the question of this study, whether we can predict the axillary pCR depending on the tumour characteristics and MRI findings. We do know the exceptional responders in the primary breast tumour to NACT including hormonal receptors (HR) negative and human epidermal growth factor receptor 2 (HER2) amplification or overexpression. We think similar predictors can be found in the axilla independent of the main tumour response.
Recent study by Ouldamer et al. (14) investigated this hypothesis and concluded that “the probability of axillary nodal pCR after NACT can be accurately predicted so that women at high probability may be spared of axillary surgery.” in our study we are looking for these predictors by investigating the tumour characteristics at diagnosis, on post NACT imaging and post-operative histopathology (HP) results. We are aiming to identify a subgroup of patients with predictable pCR after NACT. If a pCR predicting criteria is established this can help future axillary re-staging studies to even lower the FNR and have a more accurate and representative axillary staging i.e., SLNB post NACT.
Methods
A retrospective observational single center study, analysing the data collected from clinical notes and the electronic record system for all breast cancer patients with metastatic axillary LNs who underwent NACT followed by ANC surgery between 2009 and 2017.
Inclusion criteria
- Breast cancer patients with node positive disease who underwent NACT followed by axillary lymph nodes dissection in our institution from 2009 to 2017.
Exclusion criteria
- Extra nodal metastatic disease;
- Where no surgical intervention performed for any reason i.e., fitness, referral to different institution;
- Incompletion of NACT due to disease progression;
- Neo-adjuvant therapy in the form of endocrine treatment (ET);
- ANC in a node negative axilla (old practice).
Data collected
- Type of breast and axillary operation, number of LNs removed, number of the involved LNs, number of the LNs that showed treatment effect i.e., fibrosis, scarring etc.;
- Cancer characteristics; type, grade, size at diagnosis clinically and radiologically (including mammogram, US scan, and MRI), size in the final HP, presence of lympho-vascular invasion (LVI), presence of carcinoma in situ (DCIS/LCIS);
- Complete, poor, or almost complete response. While others used percentages like 80% or 100% response. Trying to have a unified system for the data analysis might have created some bias.
The total number of patients who underwent up front neoadjuvant treatment from 2009 to 2017 was 180 patients, 77 patients were excluded after applying the exclusion criteria.
The final sample size was 103 patients; all of which finished their NACT and underwent their breast and axillary surgery at our institution (Table 1).
Table 1
| Number of patients | Exclusion criteria | Patients excluded | Final sample size |
|---|---|---|---|
| 180 | Endocrine treatment | 24 | |
| 156 | Advanced disease | 11 | |
| 145 | Disease progression | 5 | |
| 140 | Reaction to NACT | 1 | |
| 139 | Referred to other facility | 1 | |
| 138 | ANC on a benign axilla | 6 | |
| 132 | ANS/SNLB on a benign xilla | 29 | 103 |
NACT, neo-adjuvant chemotherapy; ANC, axillary node clearance; SNLB, sentinel lymph node biopsy; ANS, axillary node sampling.
Data analysis
The test used is contingency table for independent sample X2 test association with confidence interval 95%. Analysis done using R. Citation: R Core Team [2018]. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/.
Ethical approval was not needed the study data was collected retrospectively with no intervention or changes to routine practice.
Results
Patients’ characteristics
Total of 180 patients received NACT from 2009 to 2017; 103/180 were eligible to be included in our study after applying the exclusion criteria (Table 1). Patients were all females between the age of 27 to 78 at the time of the diagnosis (mean 51.6 years). About 82% (84/103) of the patients’ final HP showed breast cancer of no specific type (NST/IDC), followed by invasive lobular cancer (ILC) 7/103 (6.8%)
One hundred and nine patients underwent ANC, of which 103 showed an involved axillary LNs in their final histology in the form of treatment response or residual tumour. pCR was seen in 30/103 (29%), those patients could have avoided the ANC if their response was predicted pre-operatively.
Factors associated with the outcome
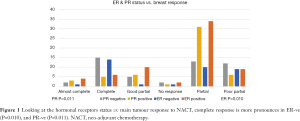
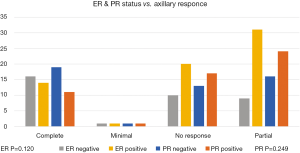
The primary breast tumour—not the axilla—response was assessed in relation to its HR status (Oestrogen ER, and Progesterone PR). A statistically significant association was found, where ER and PR negative tumours showed a higher rate of pCR when compared to ER and/or PR positive tumours (Figure 1) P values (ER 0.010, PR 0.011). Same association with the HR status was assessed in the axillary LNs response to NACT, we observed similar trend but was statistically insignificant in our sample with P values of (ER 0.120, PR 0.249) (Figure 2).
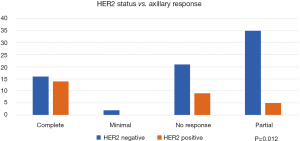
Human epidermal growth factor over expression (HER2 positive) tumour status has shown a statistically significant association with the axillary response to NACT where 14/28 (50%) of them showed axillary pCR (Figure 3), while in HER2 negative tumours 74/103 (72%), only 16/74 (21.6%) showed pCR (P=0.012). HER2 status was not documented in one case.
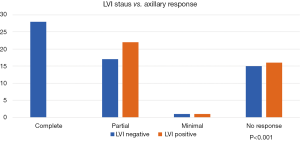
The effect of the presence or absence of LVI on the axillary response to NACT was also investigated and showed a significant association. All the patients who attained pCR in the axilla were LVI negative while none of the LVI positive patients showed a complete response (P<0.001) (Figure 4).
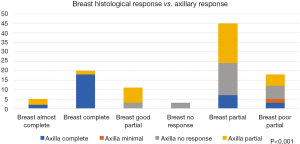
Furthermore, comparing main tumour response to the axillary LNs HP response, showed a very significant association (Figure 5), we can see that most of the axillary LNs pCR are lying within the breast pCR and pathological almost complete response bars in the histogram (P<0.001). On the other hand, 12 out of the 29 cases who had axillary pCR showed a partial response in their breast primary tumour.
Post NACT MRI scan correlation to the pathological response was assessed in the 59 patients using correlation matrix, findings were statistically insignificant with low correlation factors and insignificant P values.
Discussion
The aim of this study is to identify significant correlations between the breast tumour characteristics and the axillary pCR to NACT. This association will help identifying a subgroup of patients who might benefit from re-staging procedure and de-escalation of the axillary surgery. We have already identified few tumour factors that would help predicting the conversion of clinically node positive patients to pathologically node negative patients post NACT including HER2 positive, LVI negative and those who achieved radiological complete response in the breast. These findings were statistically significant.
As alluded to, with the on-going advances in neo-adjuvant chemo and immunotherapy regimens the role of surgical excision in this subgroup of patients is becoming a confirmation of pCR in the breast, and more limited excisions are used specially with the available response predictors in the literature like T1-2, ER negative and HER2 positive patients (15).
Some authors even went to the extreme suggesting that with the modern targeted therapies, breast surgery might be avoided completely or replaced by vacuum assisted biopsy after complete radiological response in the exceptional responders group. Such an approach should be practised cautiously as its still being examined by many trials, many surgeons felt this might be still a premature practice with recent data suggesting higher local recurrence rates in NACT with BCS (16,17).
While de-escalation of breast surgery post NACT has been very topical recently, axillary surgery has always been slow to catch up, ANC is still the gold standard procedure for metastatic axillae. Recently the concept of restaging the axilla post NACT became the subject of many trials, however there was reluctance in performing SLNB following NACT due to the high false negative rate FNR up to 12.6% in (ACOSOG)Z1071 trial, and a higher FNR of 14.2% quoted in (SENTINA) study which can be lowered to 7.3% by removing 3 or more LNs (12).
Developing a more accurate way of evaluating the axilla after NACT was a must, hence the introduction of the TAD where a marker is placed in the suspicious LN at the time of the core biopsy, then targeting the marked node plus the SLN at the time of surgery. This new technique is still under evaluation to try and find the perfect method to guide the targeted dissection; including radio-active seeds, localization wire, tattooing dyes and possibly magnetic seeds.
We looked at NACT indications in our institution from 2009 to 2017, there has been a change in pattern in the recent years. Most of the earlier patients received NACT for down staging of the cancer, fewer patients had their chemotherapy for downsizing the breast tumour to allow BCS option, and very few patients received it for tumour biology until 2016 (0–1 patients per year). However, recently more patients are receiving NACT either due to the cancer biology (e.g., triple negative, ER-ve HER2+ve), or young patients (high-risk). In 2017, 7/32 patients had NACT for their tumour biology/high-risk group, while only 6/32 patients were indicated for tumour down-sizing, this new pattern is more likely to be the trend seen in the future with a surge in the biological indication.
Although we do not have a local written NACT protocol we do follow the Midlands and east Cancer Alliance guideline (18). Breast cancer biomarkers are very important for patients and chemotherapy regimen selection because they do help in predicting the response of the main breast tumour to the chemotherapy. HR status is a well-known factor, those with negative HR are expected to have pCR more than those with HR positive tumours (19).
This was confirmed in our analysis, the pCR in the breast tissue was more in ER negative cancers (P=0.010) and in PR negative (P=0.011) than HR positive cancers. When we tested the effect of the NACT on the axillary response in HR negative cancers, once again more complete pCR in the axilla was noted in the ER and PR negative arm, but these findings were statistically insignificant with P values of 0.120 and 0.249 respectively. This statistical insignificance in this group of patients is perhaps related to the relatively small number of patients and further investigation with larger sample size is required, where we can achieve a significant correlation and avoid a type 2 statistical error, especially with the proven strong correlation between the breast main tumour pCR and the axillary LNs pCR in our study as seen in (Figure 5) which yielded a highly significant association (P<0.001), there was 20/103 cases who showed pCR in the breast, 18 out of these 20 had a pCR in the axilla; while 12 patients with pCR in the axilla had a partial response in the main tumour, this finding supports our idea of investigating the axillary pCR predictors as a separate entity.
Another tumour biomarker is HER2 over-expression that can be found in 18–20% of breast cancers, and/or HER2 amplification which is found in 5–30% (20). Those patients who achieve a pCR after NACT with anti HER2 drugs also showed a better disease free and overall survival compared to those without anti HER2 treatment. Better responses and pCR are to be expected in HER2 positive with HR negative tumours (21). As expected, our study has shown statistically significant correlation between the HER2 status and the response in the axilla (P=0.012), Figure 3 shows that 50% of HER2 positive patient achieved a PCR in the axilla versus 21% of HER2 negative patients.
Lympho-vascular invasion was investigated before as an independent prognostic factor, worse outcome after NACT was associated with LVI positive tumours (22). We reviewed the post-operative HP reports of all patients, and one of the early patterns noticed is that in many of the axillary pCR specimens there was no lympho-vascular invasion. This observation was confirmed in the final analysis that showed all patients who achieved pCR in the axilla were LVI negative, this correlation was statistically very highly significant with a P value of <0.001.
In the literature some studies evaluated the role of the MRI scans in predicting the outcome of the NACT; they reported insignificant correlation (2,3). Similarly, our study showed no statistically significant correlation between the MRI predication of the NACT effect and the actual pCR in the final HP. Therefore, MRI scan may not be used independently to predict the response in the breast or the axilla, but it might be of assistance when combined to other factors noted in this study i.e., LVI negative, HR negative, and HER2 positive cancers to form the base for a prospective trial with a larger sample size.
This study has addressed the set aims and showed some significant findings that can help in further prospective trials. At the same time there are some limitations and difficulties that necessitate being cautious in generalizing these results, we realise this is a single centre study with small number of patients, as well as the change in practice over the years that might have had an impact on the outcomes.
Conclusions
The findings of this study are relevant to our current practice and can help in suggesting a criteria for predicting pCR in the axilla, this criteria can help in designing future studies to investigate re-staging positive axillae post NACT with an accurate tool which carries a low FNR.
Our data confirmed the general trend towards giving more NACT with more indications being incorporated in our protocols. There was significant tumour characteristics that we believe can have a role in predicting patients who may attain pCR in the axilla. The main predictors were LVI which was absent in all pCR axillary LNs (P=0.001), pCR in the breast (P<0.001), HER2 positive patients (P=0.012) and finally HR negative patients (P=0.249). No significant correlation was found between the MRI as a monitoring tool and the axillary PCR.
With the limitations of this study we were able to suggest a subgroup with predictable axillary pCR. However, further randomised prospective trials are needed to validate these findings before fully adopting them in the clinical practice.
Acknowledgments
We are particularly grateful for the assistance given by the breast unit pathway co-ordinator Nikki Milburn, the records assistant Melanie Mansell, and Mr. Allen Rousell, the Cancer MDT Coordinator Lead.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2019.08.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional ethical approval and individual informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997;15:2483-93. [Crossref] [PubMed]
- Weber JJ, Jochelson MS, Eaton A, et al. MRI and Prediction of Pathologic Complete Response in the Breast and Axilla after Neoadjuvant Chemotherapy for Breast Cancer. J Am Coll Surg 2017;225:740-6. [Crossref] [PubMed]
- Hieken TJ, Boughey JC, Jones KN, et al. Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Ann Surg Oncol 2013;20:3199-204. [Crossref] [PubMed]
- Yamamoto D, Tanaka K, Tsubota Y, et al. Five-year follow-up of treatment outcomes in patients with early-stage breast cancer and clinically negative axillary nodes treated with no lymph node dissection or axillary clearance. Breast Cancer (Dove Med Press) 2012;4:125-9. [Crossref] [PubMed]
- McWhirter R. The value of simple mastectomy and radiotherapy in the treatment of cancer of the breast. Br J Radiol 1948;21:599-610. [Crossref] [PubMed]
- Payne WS, Taylor WF, Khonsari S, et al. Surgical treatment of breast cancer. Trends and factors affecting survival. Arch Surg 1970;101:105-13. [Crossref] [PubMed]
- Giuliano AE. Sentinel lymphadenectomy in primary breast carcinoma: an alternative to routine axillary dissection. J Surg Oncol 1996;62:75-7. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 1997;349:1864-7. [Crossref] [PubMed]
- Giuliano AE, Hunt K, Ballman KV, et al. Ten-year survival results of ACOSOG Z0011: A randomized trial of axillary node dissection in women with clinical T1-2 N0 M0 breast cancer who have a positive sentinel node (Alliance). J Clin Oncol 2018;34:1007. [Crossref]
- Caudle AS, Yang WT, Krishnamurthy S, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol 2016;34:1072-8. [Crossref] [PubMed]
- Rutgers EJ, Donker M, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer patients: Final analysis of the EORTC AMAROS trial (10981/22023). J Clin Oncol 2017;31:lba1001 [Crossref]
- Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013;14:609-18. [Crossref] [PubMed]
- Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455-61. [Crossref] [PubMed]
- Ouldamer L, Chas M, Arbion F, et al. Risk scoring system for predicting axillary response after neoadjuvant chemotherapy in initially node-positive women with breast cancer. Surg Oncol 2018;27:158-65. [Crossref] [PubMed]
- Swain M, Namini SN, Al-Rashdan A, et al. Predictors of Pathological Complete Response and Outcome in HER2 Positive Breast Cancer Patients Treated With Neoadjuvant Systemic Therapy, Surgery, and Radiation Therapy. Int J Radiat Oncol 2017;99:E49 [Crossref]
- Apte A, Pennell L, Chandrasekahran S. Can surgery be avoided in select breast cancer patients with complete radiological response to neoadjuvant chemotherapy? Eur J Surg Oncol 2018;44:684. [Crossref]
- Heil J, Sinn P, Richter H, et al. RESPONDER - diagnosis of pathological complete response by vacuum-assisted biopsy after neoadjuvant chemotherapy in breast Cancer - a multicenter, confirmative, one-armed, intra-individually-controlled, open, diagnostic trial. BMC Cancer 2018;18:851. [Crossref] [PubMed]
- Available online: https://www.england.nhs.uk/midlands/wp-content/uploads/sites/46/2019/05/guidelines-for-the-management-of-breast-cancer-v1.pdf
- Connolly RM, Stearns V. Current approaches for neoadjuvant chemotherapy in breast cancer. Eur J Pharmacol 2013;717:58-66. [Crossref] [PubMed]
- Parakh S, Gan HK, Parslow AC, et al. Evolution of anti-HER2 therapies for cancer treatment. Cancer Treat Rev 2017;59:1-21. [Crossref] [PubMed]
- Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol 2012;30:3242-9. [Crossref] [PubMed]
- Liu YL, Saraf A, Lee SM, et al. Lymphovascular invasion is an independent predictor of survival in breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 2016;157:555-64. [Crossref] [PubMed]
Cite this article as: Elamin G, Sapre D, Tehniyat W, Jahan A, Dakka M. Pathological complete response in the axillary lymph nodes post neo-adjuvant chemotherapy in breast cancer, is it predictable? Ann Breast Surg 2019;3:20.