In patients with micrometastases in sentinel lymph node biopsies, involvement of the non-sentinel lymph nodes cannot be predicted by clinicopathological variables
Introduction
Each year, about 54,900 women in the UK are diagnosed with breast cancer, that’s around 150 every day and the most of them (81%) undergo surgery (1). There is strong evidence that in the early stages of breast cancer, sentinel lymph node biopsy (SLNB) can accurately stage the axillary disease leading to low axillary recurrence rates, comparable survival and reduced morbidity when compared with axillary dissection (2,3).
SLNB has showed its effectiveness in reducing the risk of lymphoedema, shoulder pain, sensory deficits, and surgical site infection than axillary node clearance (ANC). Quality of life is also found to be superior in patients who undergo SLNB only (4). These results have led this technique to become a treatment of choice and has become a standard technique around the world and the Department of Health in the UK (5).
It is now an accepted rule that patients who have positive sentinel lymph node (SLN) require further treatment either Axillary node clearance or Radiotherapy. But patients whose SLNB is negative do not require any further axillary specific treatment.
The current UK National Institute for Health and Care Excellence (NICE) guidelines (6) recommend that ANC or axillary radiotherapy (ART) for women with early stage breast cancer with one or two positive sentinel nodes. This recommendation assumes that axillary treatment with surgery and or radiotherapy reduces the risk of axillary recurrence and can increase the chances of survival. Axillary node clearance is usually done as a second stage procedure.
Due to recent advances in Breast cancer investigations and patient awareness, 60–70% of patients are early breast cancer which are node negative at the time of diagnosis (7). Axillary surgery affects the lymphatic drainage from the arm and exposes patients to risk of both short- and long-term morbidities (8). Completion axillary lymph node dissection (CALND) is associated with an overall complication rates of 20–30% according to literature which includes seroma formation, local swelling, numbness of the arm and shoulder, impaired shoulder movement, neuropathy, surgical site infection, and chronic lymphoedema (9).
These complications of axillary surgery are very distressing which impairs the quality of life and daily activities. They have financial implications to the NHS in terms of rehabilitative treatments as they are often irreversible and symptom relief is not achievable in most of the cases.
The incidence of non-SLN involvement changes considerably with the extent of disease in the SLNB. The results of studies have shown that 53% of patients with a positive SLNB were found to have disease in non-SLNs (10). For patients whose SLNB was involved by macro metastatic disease (tumour metastases greater than 2 mm), the incidence of non-SLN involvement is reported to be 40% to 58% (11). When the SLN is involved by micrometastatic disease (nodal metastasis 0.2 to 2 mm), the incidence of non-SLN involvement is 20% (12) and in the case of the SLN with isolated tumour cells (ITC) (=0.2 mm), the incidence decreases to 12% (13).
Factors that influence the degree of non-SLN involvement are histology and grade of tumour, tumour size, multifocality, lympho-vascular invasion, estrogen receptor (ER) status, and the ratio of positive SLNs to the total number of sentinel nodes removed (14-16). These findings have played significant role in developing trends for doing CALND in certain patients, particularly those thought to be at risk of having additional disease in the non-SLNs.
The American Society of Breast Surgeons issued a consensus statement in 2005 acknowledging this trend (17): “Outside of clinical trials, usual treatment for SLN-positive patients is a level I-II ALND. However, since axillary node metastases are limited to the SLN in more than half of SLN-positive individuals, there may be low-risk subsets for whom a completion ALND is not required. The decision to omit completion axillary dissection in such a case requires a balanced discussion between the surgeon and the patient regarding the risks of further surgery and any potential for improved outcome with more complete information and/or axillary clearance.”
The SLN has been demonstrated to be the only positive lymph node in many cases. Data from high-volume breast cancer centres indicate that the SLN is the only site of metastases in 40% to 60% of axillary dissections (18).
From the above discussion we can conclude that future of SLNB and CALND is dependent on the assessment of risk factors which can lead to prediction of non-SLN positivity or negativity, which will lead to avoidance of CALND and its related morbidities. Many Normograms have already been developed to predict this. But we think the future of breast cancer management of the axilla lies in a prediction tool for non-SLN biopsy.
Considering the above discussion and unanswered questions about CALND, we decided to conduct this study to identify clinicopathological variables which can predict the involvement of non-SLN.
Methods
We retrospectively analysed our experience of SNLB between July 2008 and July 2013. A total of 1,152 breast cancer patients underwent SLNB.
This is a retrospective analysis in a single institution. Data on procedures performed was prospectively collected by the theatre admin team, and once patient details were identified, the electronic notes and results were searched to obtain further data retrospectively for this study.
We looked for the clinicopathological variables to predict non-SLN status in SLNB with micrometastasis & macrometastasis. The variables were tumour grade, size of primary breast tumour, lymphovascular invasion, age and number of positive SLNB (nearly all patients had <2 positive nodes on SLNB).
Surgery and procedure
A total of 1,152 breast cancer patients underwent SLNB based on lymphoscintigraphy, intra-operative gamma probe detection, and blue dye mapping using 99m Tc-nanocolloid and Patent Blue V injected peri-areola. We used dual method blue dye and 99m Tc-nanocolloid due to higher accuracy.
Statistical analysis was performed using Fisher’s exact and χ2 for categorical data. All data analysis was performed using SPSS version 20 for mac. All reported P values are two-sided.
Pathological evaluation
Micrometastases, macrometastases and ITC were classified as per AJCC 6th Edition.
Tumour deposits measuring 2 mm or more were classified as macrometastases. Tumour deposits measuring 0.2 to 2 mm were classified as micrometastases. Tumour deposits measuring less than 0.2 mm were classified as ITC.
Immunohistochemistry for ER, progesterone receptor (PR) and human epidermal growth factor receptor 2 (Her2) are all performed and interpreted in our department. For this study, ER and PR Quickscore of 4 or more was considered positive. However, current guidelines for ER and PR state that they should be considered positive if 1% or more of tumour cell nuclei are positive.
For Her2, an immunohistochemistry score of 0 or 1+ is negative and a score of 3+ is positive. A score of 2+ is considered borderline and is therefore referred to Leeds Teaching Hospitals NHS Trust for fluorescent in situ hybridization (FISH) analysis.
Results
Out of 1,152 SLNB biopsies performed, 224 (19.4%) were positive for metastatic disease which includes macrometastases in 150 (67.0%), micrometastases in 72 (32.1%) and ITC in 2 (0.9%) (Table 1).
Table 1
| Total SLNB | Total + Ve (%) | Macrometastases (%) | Micrometastases (%) | ITC (%) |
|---|---|---|---|---|
| 1,152 | 224 (19.4) | 150 (67.0) | 72 (32.1) | 2 (0.9) |
SLNB, sentinel lymph node biopsy; Ve, positive; ITC, isolated tumour cells.
Types of primary breast cancer in all SLNB positive patients invasive ductal carcinoma (IDC) =84%, Mixed type 9%, Lobular 4.74%, Others 1.74%, Tubular 0.43%.
Types of cancer in micrometastasis group only Ductal 85.5%, Lobular 4.8%, Tubular 3.2%, Mixed 4.8%, Others 1.6%. CALND was not performed in 20 cases (9 macrometastases, 10 micrometastases, and 1 ITC), largely due to concerns regarding fitness for anaesthesia.
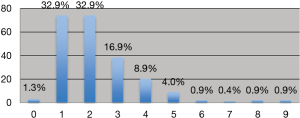
Mastectomies =53%, breast conserving surgeries (wide local excision) =47%. About 65.8% 1–2 SLN were removed (Figure 1).
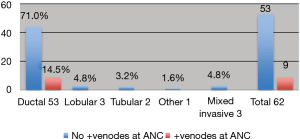
Primary cancer was IDC in 85.5% (53/62) of cases in group and 9/62 with micrometastases had n-SNB positive on CALND. In micrometastases all positive nodes at ANC belong IDC group i.e., 17.0% (9/53) (Figure 2).
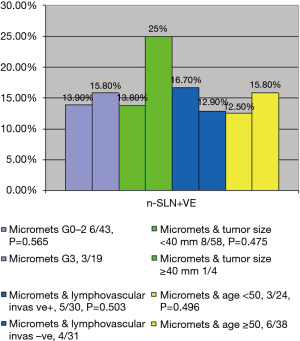
On univariate analysis, positive non-SLN in CALND for patients with micrometastases on SLNB was not predicted by grade (G0–G2, 6/43; G3, 3/19; P=0.565), size of primary breast tumour (<40 mm, 8/58; ≥40 mm, 1/4; P=0.475), lympho-vascular invasion (5/30 vs. 4/31, P=0.503), age (<50 years, 3/24 vs. ≥50 years, 6/38; P=0.496), or number of positive SLNB (all patients had <2 positive nodes on SLNB) (Figure 3).
Macrometastases on SNLB were more likely to predict positive non-SLN on ANC [macrometastases; 39/141(27.7%) vs. micrometastases; 9/62 (14.5%), P=0.029] (Table 2).
Table 2
| Characteristics | Lymph node +Ve | Lymph node -Ve | P value |
|---|---|---|---|
| Age | |||
| <50 | 3/24 | 21/24 | 0.496 |
| ≥50 | 6/38 | 32/38 | |
| Grade | |||
| 0-2 | 6/43 | 37/43 | 0.565 |
| 3 | 3/19 | 16/19 | |
| Tumour size | |||
| <40 mm | 8/58 | 50/58 | 0.475 |
| ≥40 mm | 1/4 | 3/4 | |
| LV invasion | |||
| +Ve | 5/30 | 25/30 | 0.503 |
| ‒Ve | 4/31 | 27/31 | |
| Micrometastases +Ve (%) | 9/62 (14.5) | ||
| Macrometastases +Ve (%) | 39/141 (27.7) | 0.029 |
SLN, sentinel lymph node; Ve, positive; LV, lymphovascular invasion.
Discussion
The American Joint Committee on Cancer (AJCC) defined a lymph node metastatic tumour with maximum diameter of >2 mm as macrometastases (pN1) while the diameter of deposit is 0.2–2 mm as micrometastases (pNmi). The lesion of single tumour cells or small cell clusters with diameter <0.2 mm is defined as ITCs [pN0(i+)] (19).
The management of patients with minimal SLN involvement is challenging and has been an increasingly important question since the start of SLNB.
Since the recognition of terminologies of macrometastases, micrometastases, and ITC it has prompted research on the management of these conditions as well. As far as literature is concerned there are studies which support and oppose further treatment with micro and macrometastases.
In our study, clinicopathological variables (age, size of tumour, Grade & lymphovascular involvement) of micrometastases have not predicted the involvement of non-SLN. However, we found that 14.5% of patients with micrometastases on SLNB may have positive non-SLN which is important because if CALND is not being undertaken in these patients, patients must be informed about the chance of having further positive nodes in remaining Axilla.
AMAROS explores the benefit of a CALND vs. ART in patients with SLN-positive breast cancer (20). A sub study investigated the identification rate and the nodal involvement of the first 2,000 patients, data was collected for 4 years from 26 European institutions (20). The sentinel node identification rate was 97%. Total of 34% SLN were positive of whom 63% had macrometastases, 25% had micrometastases, and 12% had ITCs. In patients with complete axillary node dissection non-SLN involvement was found to be 41% with macrometastases and 18% had either micrometastases or ITCs.
Many researchers have probed the incidence of non-SLN involvement in patients with SLN micrometastases to characterise which group of patients should get further axillary treatment. Wada and Imoto accumulated 22 studies from 1999 to 2006 related to the frequency of SLN micrometastases in patients with breast cancer and the rate of non-SLN involvement in those patients after ALND (21). The count of SLN micrometastases was 38% with non-SLN micrometastases ranged from 0 to 57%. Moreover, a wide range of non-SLN macrometastases was found (0–18%). As the count of non-SLN micrometastases was low, the prognostic impact was uncertain. Most of the studies had small numbers and short follow up and concluded that there is no benefit from CALND. The largest study; however, found a significantly worse disease-free survival for women with micrometastases who did not undergo CALND (22).
In the largest published multicenter retrospective study of 187 SLN-ITCs patients undergoing CALND, Houvenaeghel et al. found an incidence of 16% non-SLN involvement (23,24). The difference in the risk of non-SLN involvement between sentinel nodes with ITCs (16%) and those with micrometastases (14%) was not statistically significant. However, it was not clear that whether the presence of non-SLN metastases should affect the treatment decision in these patients. In contradiction to the above conclusions comes the MIRROR trial results (25). MIRROR was a large Dutch cohort retrospective study which evaluated the effect of SLN-ITCs and micrometastases on 5-year disease free survival in patients with favourable primary tumour characteristics.
Patients with SNB micrometastases and those who have ITCs who did not undergo CALND had a higher 5-year axillary recurrence rate, 6% in comparison to 1% of SNB negative patients who did not undergo CALND. Moreover, patients with SLN micrometastases and ITCs had a higher 5-year disease-free survival improved by 10% with adjuvant systemic therapy. Pertinent to mention that micrometastases and ITCs had comparable prognostic impact (26). MIRROR results recommended an aggressive approach for treatment in patients with either SLN micrometastases or ITCs.
Lastly it will be interesting to know the outcome of POSNOC trial in connection with management of the Axilla in early breast cancer. POSNOC study design has two arms, one arm with adjuvant therapy but no treatment to axilla after surgery, while in the other arm adjuvant therapy plus treatment to axilla after surgery. Study is expected to be completed in 2024.
Conclusions
CALND in patients with micrometastases is still under debate in surgical communities. Although macrometastases have a higher predictive value to detect non-SLN involvement compared to presence of micrometastases, risk is still there that patients who have micrometastases may have positive non-SLNB. Our study suggests that patients with micrometastases should not be routinely offered axillary clearance but should be informed of the small risk of having non-SLN involvement which is not predicted by any clinicopathological characteristics.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2019.08.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was not required as anonymous data is used in compliance with NHS Data Act 2006. Individual informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Breast cancer statistics. Cancer Research UK. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer#heading-Zero
- Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003;349:546-53. [Crossref] [PubMed]
- Gill GSNAC Trial Group of the Royal Australasian College of Surgeons (RACS) and NHMRC Clinical Trials Centre. Sentinel-lymph-node-based management or routine axillary clearance? One-year outcomes of sentinel node biopsy versus axillary clearance (SNAC): a randomized controlled surgical trial. Ann Surg Oncol 2009;16:266-75. [Crossref] [PubMed]
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-609. [Crossref] [PubMed]
- Mansel RE, MacNeill F, Horgan K, et al. Results of a national training programme in sentinel lymph node biopsy for breast cancer. Br J Surg 2013;100:654-61. [Crossref] [PubMed]
- Early and locally advanced breast cancer: diagnosis and treatment. Available online: http://guidance nice org uk/CG80
- Kell MR, Kerin MJ. Sentinel lymph node biopsy. BMJ 2004;328:1330-1. [Crossref] [PubMed]
- Cady B, Stone MD, Schuler JG, et al. The new era in breast cancer. Invasion, size, and nodal involvement dramatically decreasing as a result of mammographic screening. Arch Surg 1996;131:301-8. [Crossref] [PubMed]
- Giuliano AE, Haigh PI, Brennan MB, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol 2000;18:2553-9. [Crossref] [PubMed]
- Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer 2006;106:4-16. [Crossref] [PubMed]
- Kamath VJ, Giuliano R, Dauway EL, et al. Characteristics of the sentinel lymph node in breast cancer predict further involvement of higher-echelon nodes in the axilla: a study to evaluate the need for complete axillary lymph node dissection. Arch Surg 2001;136:688-92. [Crossref] [PubMed]
- Cserni G. Sentinel lymph-node biopsy-based prediction of further breast cancer metastases in the axilla. Eur J Surg Oncol 2001;27:532-8. [Crossref] [PubMed]
- van Deurzen CH, de Boer M, Monninkhof EM, et al. Non-sentinel lymph node metastases associated with isolated breast cancer cells in the sentinel node. J Natl Cancer Inst 2008;100:1574-80. [Crossref] [PubMed]
- Hwang RF, Gonzalez-Angulo AM, Yi M, et al. Low locoregional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer 2007;110:723-30. [Crossref] [PubMed]
- Tan EY, Ho B, Chen JJ, et al. Predictors of nonsentinel nodal involvement to aid intraoperative decision making in breast cancer patients with positive sentinel lymph nodes. ISRN Oncol 2011;2011:539503 [Crossref] [PubMed]
- Park J, Fey JV, Naik AM, et al. A declining rate of completion axillary dissection in sentinel lymph node-positive breast cancer patients is associated with the use of a multivariate nomogram. Ann Surg 2007;245:462-8. [Crossref] [PubMed]
- The American Society of Breast Surgeons. Official Statements. Approved December 5, 2008. Accessed April 22, 2012.Available online: https://www.breastsurgeons.org/resources/statements
- Grube BJ, Giuliano AE. Observation of the breast cancer patient with a tumor-positive sentinel node: implications of the ACOSOG Z0011 trial. Semin Surg Oncol 2001;20:230-7. [Crossref] [PubMed]
- Singletary SE, Greene FL, Breast Task Force. Revision of breast cancer staging: the 6th edition of the TNM Classification. Semin Surg Oncol 2003;21:53-9.
- Straver ME, Meijnen P, van Tienhoven G, et al. Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol 2010;17:1854-61. [Crossref] [PubMed]
- Wada N, Imoto S. Clinical evidence of breast cancer micrometastasis in the era of sentinel node biopsy. Int J Clin Oncol 2008;13:24-32. [Crossref] [PubMed]
- Reed J, Rosman M, Verbanac KM, et al. Prognostic implications of isolated tumor cells and micrometastases in sentinel nodes of patients with invasive breast cancer: 10-year analysis of patients enrolled in the prospective East Carolina University/Anne Arundel Medical Center Sentinel Node Multicenter Study. J Am Coll Surg 2009;208:333-40. [Crossref] [PubMed]
- Houvenaeghel G, Nos C, Mignotte H, et al. Micrometastases in sentinel lymph node in a multicentric study: predictive factors of nonsentinel lymph node involvement--Groupe des Chirurgiens de la Federation des Centres de Lutte Contre le Cancer. J Clin Oncol 2006;24:1814-22. [Crossref] [PubMed]
- Pazaiti A, Fentiman IS. Which Patients Need an Axillary Clearance after Sentinel Node Biopsy? Int J Breast Cancer 2011; [Crossref] [PubMed]
- Tjan-Heijnen VC, de Boer M. Minimal lymph node involvement and outcome of breast cancer. The results of the Dutch MIRROR study. Discov Med 2009;8:137-9. [PubMed]
- Tjan-Heijnen VC, Pepels MJ, de Boer M, et al. Impact of omission of completion axillary lymph node dissection (cALND) or axillary radiotherapy (ax RT) in breast cancer patients with micrometastases (pN1mi) or isolated tumor cells (pN0[i+]) in the sentinel lymph node (SN): Results from the MIRROR study. J Clin Oncol 2016; [Crossref]
Cite this article as: Chauhan MN, Majeed T, Ghaus M, Dev R, Ahmed S, Sherpa S, Sayers C, Kryjack Z, Ali D. In patients with micrometastases in sentinel lymph node biopsies, involvement of the non-sentinel lymph nodes cannot be predicted by clinicopathological variables. Ann Breast Surg 2019;3:21.