
Do all BRCA1/2 carriers with breast cancer benefit from bilateral mastectomy?
Which breast cancer patients should undergo BRCA testing?
Since the discovery of the BRCA1 (1) and BRCA2 genes (2) over 25 years ago, understanding and management of hereditary breast and ovarian cancer patients have been constantly evolving. In the general population BRCA1/2 mutations occur in 1 every 300 to 500 women and account for 5% and 15% of breast and ovarian cancer cases, respectively. BRCA1 and BRCA2 carriers exhibit a similar cumulative breast cancer risk to the age of 80 (72% and 69%, respectively), while both ovarian cancer risk (44% vs. 17%) and contralateral cumulative breast risk 20 years after breast cancer diagnosis (40% vs. 26%) are higher for BRCA1 vs. BRCA2 carriers (3). Given the substantial risk of subsequent breast and ovarian cancers, knowledge of BRCA1/2 mutation status may be important when cancer develops as it may influence the attitude of patients towards surveillance and preventive options (4).
The prevalence of BRCA pathogenic variants in unselected breast cancer patients is less than 2%, yet up to 60% of carriers are not diagnosed according to current criteria of access to genetic testing (5). This gap of knowledge is becoming a critical issue in breast cancer care. Indeed, the relevance of BRCA status is increasing not only from the surgical standpoint, but also to tailor medical therapies as it predicts responsiveness to platinum-based chemotherapy and poly(ADP-ribose) polymerase (PARP) inhibitors (6). Therefore, universal genetic testing for ovarian cancer patients and a broadening of criteria to genetic testing for breast cancer patients have been suggested (7). This change of practice is reflected in the recently published US Preventive Services Task Force statement on genetic testing for BRCA-related cancer (8).
Neoadjuvant chemotherapy vs. primary surgery
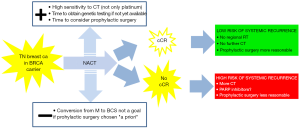
The available scientific evidence on breast cancer overall survival of BRCA carriers as compared to non-carriers is conflicting, although large differences are unlikely to exist (Table 1) (9-14). A recently published prospective study assessed the relationship between germ-line BRCA mutation and outcome in a large cohort of young-onset breast cancer patients in the UK (15). Overall, patients with a BRCA mutation had similar survival as non-carriers [hazard ratio (HR) 0.96, 95% confidence interval (CI): 0.76–1.22; P=0.76]; nevertheless, carriers with triple-negative (TN) breast cancer had a survival advantage during the first few years after diagnosis compared with non-carriers (HR 0.59, 95% CI: 0.35–0.99; P=0.047), likely due to the greater sensitivity of BRCA-mutant breast cancers to chemotherapy. Indeed, a systematic review and meta-analysis by Caramelo et al. suggests that the addition of platinum to chemotherapy regimens in the neoadjuvant setting increases the complete pathological response (pCR) rate in BRCA-mutated as compared to wild-type TNBC patients (16). Neoadjuvant chemotherapy (NACT) is particularly attractive in suspected BRCA carriers with TN tumors primarily due the expected chemosensitivity of their tumors. In addition, NACT provides time to get the result of genetic testing if not yet available, and to consider pros and cons of prophylactic surgery if the test is positive. Furthermore, tumor response may help to tailor further adjuvant regional and systemic treatments, as well as the opportunity to undergo prophylactic surgery based on the expected outcome of the patient (Figure 1).
Table 1
| Author, year | Main results |
|---|---|
| Lee, 2010 (9) | • BRCA1 mutation decreases short-term and long-term OS and short-term PFS |
| • BRCA2 mutation does not affect either short-term or long-term survival rate | |
| Zhong, 2015 (10) | • Among patients with breast cancer, BRCA1 mutation carriers had worse OS than non-carriers |
| • BRCA2 mutation was not associated with breast cancer prognosis | |
| van den Broek, 2015 (11) | • Current evidence does not support worse breast cancer survival of BRCA1/2 carriers |
| Templeton, 2016 (12) | • BRCA mutations were not associated with worse overall survival |
| Bernier, 2015 (13) | • There is currently no evidence that, provided adequate systemic treatment is part of the therapeutic management, significant differences in both BCSS and OS |
| Hallam, 2015 (14) | • In most studies there was no significant difference in survival for BRCA1/2 carriers |
OS, overall survival; PFS, progression free survival; BCCS, breast cancer specific survival.
Breast conserving surgery (BCS) vs. mastectomy
The role of breast conserving surgery followed by radiotherapy (RT) in BRCA carriers has long been debated. It has been suggested that, as BRCA mutations may predispose carriers to higher cellular sensitivity to ionizing radiation, tumors responses may be better, but toxicity and radiation-induced malignancies may also be increased. Fortunately, the available data on complications are reassuring and there appears to be no increase in contralateral breast cancer (CBC) risk from scatter radiotherapy (RT) (13,17).
As tumor control is concerned, most studies do not show significant differences of ipsilateral breast tumor recurrences (IBTR) in BRCA carriers treated by BCS + RT as compared to non-carriers (Table 2) (17-26). The metanalysis by Valachis et al. confirms this finding [relative risk (RR) 1.45, 95% CI: 0.98–2.14], yet shows a significant higher risk for ipsilateral breast tumor recurrences (IBTR) among BRCA-mutation carriers in studies with a median follow-up ≥7 years (RR 1.51, 95% CI: 1.15–1.98) (27). The latter finding, coupled with the observation that BRCA carriers develop more new events elsewhere in the breast (i.e., not in the quadrant where the original tumor was located) than non-carriers (19), suggests that these “late” IBTR are likely new primaries and not local recurrences.
Table 2
| Author, year | BRCA carriers | Controls | Median FU (years) | IBTR (%) carriers | IBTR (%) controls | P |
|---|---|---|---|---|---|---|
| Pierce, 2000 (17) | 71 | 203 | 5 | 14 | 16 | 0.84 |
| Haffty, 2002 (18) | 22 | 105 | 13 | 49 | 21 | 0.007 |
| Seynaeve, 2004 (19) | 26 | 174 | 6.0 | 21.8 | 12.1 | 0.05 |
| Robson, 2005 (20) | 56 | 440 | 9.7 | 12 | 8 | 0.68 |
| Kirova, 2005 (21) | 29 | 271 | 8.8 | 24 | 19 | 0.47 |
| Pierce, 2006 (22) | 170 | 469 | 8.3 | 12.5 | 8.6 | 0.55 |
| Brekelmans, 2007 (23) | 109 | 410 | 4.3 | 12/17 | 12 | 0.6 |
| Garcia Etienne, 2009 (24) | 54 | 162 | 4.0 | 27 | 4 | 0.03 |
| Kirova, 2010 (25) | 29 | 58 | 13.4 | 36 | 33 | 0.42 |
| van den Broek, 2019 (26) | 55 | 1,510 | 12 | 7.3 | 7.9 | – |
FU, follow up; IBTR, ipsilateral breast tumor recurrence; BCS, breast conserving surgery; RT, radiotherapy.
The same explanation may underlie the increased risk of local failure in BRCA carriers treated with BCS + RT vs. mastectomy (Table 3) (26,28,29). Importantly, in none of these studies significant differences for overall survival (OS), breast cancer death, or distant recurrence have been reported according to type of surgery performed.
Table 3
| Author, year | BCS | M | Median FU (years) | BCS vs. M at 10 yrs | BCS vs. M at 15 yrs | |||
|---|---|---|---|---|---|---|---|---|
| Local failure (%) | P | Local failure (%) | P | |||||
| Pierce, 2010 (28) | 302 | 353 | 8.2/8.9 | 10.5 vs. 3.5 | 0.0001 | 23.5 vs. 5.5 | 0.0001 | |
| Nilsson, 2104 (29) | 45 | 118 | 14.9/12.1 | 25 vs. 9 | 0.03 | 32 vs. 9 | 0.03 | |
| van den Broek, 2019 (26) | 91 | 49 | – | 7.3 vs. 1.5 | – | – | – | |
FU, follow up; IBTR, ipsilateral breast tumor recurrence; BCS, breast conserving surgery; RT, radiotherapy; M, mastectomy.
Contralateral prophylactic mastectomy vs. surveillance
It has been repeatedly substantiated that the risk of CBC in BRCA1/2 carriers is significantly higher as compared to non-carriers. In the meta-analysis by Valachis et al. the number of CBCs was significantly greater in carriers versus controls (RR 3.56, 95% CI: 2.50–5.08) (27). In a more recent meta-analysis, the cumulative 5-year risk of CBC for BRCA1 and BRCA2 mutation carriers was 15% (95% CI: 9.5–20%) and 9% (95% CI: 5–14%), respectively and the 10-year risk increased up to 27% and 19%, respectively (30).
Similarly to the general population, family history of breast cancer and young age at primary breast cancer diagnosis increase CBC risk also in BRCA carriers, while endocrine therapy and risk reducing salpingo-oophorectomy (RRSO) decrease the risk (31). Somehow unexpectedly, while the type of surgery on the primary tumor (BCS vs. mastectomy) does not influence overall survival, several studies suggest that contralateral prophylactic mastectomy (CPM) may improve OS, likely trough the prevention of new contralateral tumors (Table 4) (32-35). In the most recent of such studies the mortality was lower in the CPM group than in the surveillance group (adjusted HR 0.49, 95% CI: 0.29–0.82) and the survival benefit was especially seen in patients <40 years of age, with grade 1/2 and/or no TN tumors and if were not treated with adjuvant chemotherapy (35).
Table 4
| Author | CPM/no-CPM | Mean FU (yrs) | No. CBC | OS | |||
|---|---|---|---|---|---|---|---|
| CPM vs. no-CPM (%) | P value | CPM vs. no-CPM (%) | P value | ||||
| Van Sprundel, 2005 (32) | 78/69 | 3.5 | 1 vs. 6 (1 vs. 8.7) | 0.001 | 94 vs. 77 | 0.003* | |
| Evans, 2013 (33) | 105/473 | 9.7/8.6 | 6 vs. 118 (6 vs. 25) | – | 89 vs. 71 | <0.001° | |
| Metcalfe, 2014 (34) | 181/209 | 14.3 | 1 vs. 70 (0.6 vs. 33.5) | 0.0004 | 88 vs. 66 | 0.03§ | |
| Heemskerk-Gerritsen, 2105 (35) | 242/341 | 11.4 | 4 vs. 64 (2 vs. 19) | 0.001 | 92 vs. 81 | <0.001 | |
*No longer significant after adjustment for BPO in a multivariate Cox analysis; The survival advantage remained after matching for oophorectomy, gene, grade and stage; §In a propensity score adjusted analysis of 79 matched pairs, the association was not significant (P=0.08). CPM, contralateral prophylactic mastectomy; FU, follow up; CBC, contralateral breast cancer; OS, over.
Variables in the surgical decision-making process
In BRCA carriers who develop breast cancer, similarly to all breast cancer patients, the risks of ipsilateral, contralateral and distant new events are significantly modified by several factors. With regard to local control, in patients undergoing BCT the risk of a second in-breast event is significantly reduced by chemotherapy (17). Risk reducing salpingo-oophorectomy (RRSO) has a protective effect against recurrences [hazard ratio (HR) 0.50; 95% CI: 0.31 to 0.69], but it also reduces the risk of death (HR =0.33; 95% CI: 0.28 to 0.38) (36). Also tamoxifen is associated with a reduction in CBC risk both for BRCA1 (HR 0.38, 95% CI: 0.27 to 0.55) and BRCA2 carriers (HR 0.33; 95% CI: 0.22 to 0.50) (37).
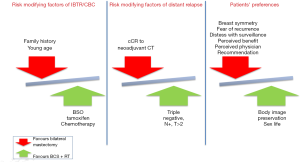
The mutation in either BRCA1 or BRCA2 and patient’s age are important factors to consider when deciding the optimal surgical approach. For example, it has been shown that 62.9% of BRCA1 carriers less than 40 years of age at first breast cancer developed a CBC vs. 19.6% of those who were older than 50 years and that BRCA1 carriers had a 1.6-fold (95% CI: 1.2- to 2.3-fold) higher risk of CBC than BRCA2 carriers after 25 years of follow up (38). The risk-benefit ratio of prophylactic surgery may also be influenced by a history of previous breast irradiation. Radiotherapy may in fact exert a preventive effect on the development of new primaries (39), but it increases the likelihood of complications and unfavorable outcomes of reconstructive surgery (40). Finally, the decision should always respect patients’ preferences, taking into account that the latter can be heavily influenced by perceived physician recommendation for CPM, greater perceived contralateral breast cancer risk, and greater perceived benefits of CPM (41,42) (Figure 2).
Conclusions
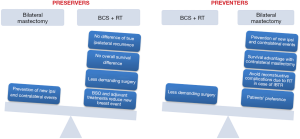
BRCA1/2 carriers who develop breast cancer face difficult decisions regarding their surgical options mainly due to their increased lifetime risk of developing other breast and ovarian cancers. In carriers, BCS and mastectomy provide the same survival, as well as in the general population, yet the risk of late new ipsilateral breast cancers after BCS is increased. This event is a source of severe psychological distress by itself and, as it requires a mastectomy, exposes these patients to higher risk of surgical complications and unfavorable reconstructive results due to the previous breast RT. Furthermore, the lifetime risk of CBC is significantly increased, especially in very young and BRCA1-positive patients. Therefore, as CPM in this setting may even improve overall survival, a thorough discussion of pros and cons of BCS + RT vs. therapeutic mastectomy and CPM is warranted in all BRCA1/2 carriers with unilateral breast cancer (Figure 3).
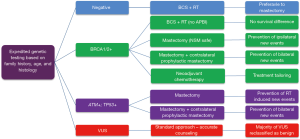
Expedited genetic testing is becoming a critical issue for many newly diagnosed breast cancer patients; fortunately, a broadening of indications to gene counseling and the rapid analysis of gene panels are now possible thanks to the introduction of next generation sequencing (NGS). A negative BRCA1/2 test may provide reassurance on the safety of BCS + RT, while limiting the unjustified increase of bilateral mastectomies that took place over the last 15 years worldwide. Conversely, the awareness of a positive test may help patients to choose their favorite surgical option and physicians to tailor neoadjuvant and adjuvant therapies. Large scale genetic testing with NGS will provide more in depth information on the risks associated with variants of unknown significant (VUS) of BRCA1/2 and with other cancer predisposing genes. Better knowledge may hopefully lead to the creation of decision-making/aiding algorithms that will better take into account the complex interplay between clinical, biological and treatment variables (Figure 4) that characterizes the care of these patients.
Acknowledgments
This study is supported by FPRC 5xmille Ministero della Salute 2015 (Strategy); FPRC 5xmille Ministero della Salute 2013; Ricerca Corrente 2019.
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2019.09.01). RP serves as an unpaid editorial board member of Annals of Breast Surgery from Aug 2019 to Jul 2021.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994;266:66-71. [Crossref] [PubMed]
- Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995;378:789-92. [Crossref] [PubMed]
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017;317:2402-16. [Crossref] [PubMed]
- Chiba A, Hoskin TL, Hallberg EJ, et al. Impact that Timing of Genetic Mutation Diagnosis has on Surgical Decision Making and Outcome for BRCA1/BRCA2 Mutation Carriers with Breast Cancer. Ann Surg Oncol 2016;23:3232-8. [Crossref] [PubMed]
- Li J, Wen WX, Eklund M, et al. Prevalence of BRCA1 and BRCA2 pathogenic variants in a large, unselected breast cancer cohort. Int J Cancer 2019;144:1195-204. [Crossref] [PubMed]
- Tung NM, Garber JE. BRCA1/2 testing: therapeutic implications for breast cancer management. Br J Cancer 2018;119:141-52. [Crossref] [PubMed]
- Vos JR, Fakkert IE, de Hullu JA, et al. Universal tumor DNA BRCA1/2 testing of ovarian cancer: prescreening PARPi treatment and genetic predisposition. J Natl Cancer Inst 2020;112:161-9. [Crossref]
- Owens DK, Davidson KW, Krist AH, et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2019;322:652-65. [Crossref] [PubMed]
- Lee EH, Park SK, Park B, et al. Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Res Treat 2010;122:11-25. [Crossref] [PubMed]
- Zhong Q, Peng HL, Zhao X, et al. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res 2015;21:211-20. [Crossref] [PubMed]
- van den Broek AJ, Schmidt MK, van't Veer LJ, et al. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what's the evidence? A systematic review with meta-analysis. PLoS One 2015;10:e0120189 [Crossref] [PubMed]
- Templeton AJ, Gonzalez LD, Vera-Badillo FE, et al. Interaction between Hormonal Receptor Status, Age and Survival in Patients with BRCA1/2 Germline Mutations: A Systematic Review and Meta-Regression. PLoS One 2016;11:e0154789 [Crossref] [PubMed]
- Bernier J, Poortmans P. Clinical relevance of normal and tumour cell radiosensitivity in BRCA1/BRCA2 mutation carriers: a review. Breast 2015;24:100-6. [Crossref] [PubMed]
- Hallam S, Govindarajulu S, Huckett B, et al. BRCA1/2 Mutation-associated Breast Cancer, Wide Local Excision and Radiotherapy or Unilateral Mastectomy: A Systematic Review. Clin Oncol (R Coll Radiol) 2015;27:527-35. [Crossref] [PubMed]
- Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol 2018;19:169-80. [Crossref] [PubMed]
- Caramelo O, Silva C, Caramelo F, et al. The effect of neoadjuvant platinum-based chemotherapy in BRCA mutated triple negative breast cancers -systematic review and meta-analysis. Hered Cancer Clin Pract 2019;17:11. [Crossref] [PubMed]
- Pierce LJ, Strawderman M, Narod SA, et al. Effect of radiotherapy after breast-conserving treatment in women with breast cancer and germline BRCA1/2 mutations. J Clin Oncol 2000;18:3360-9. [Crossref] [PubMed]
- Haffty BG, Harrold E, Khan AJ, et al. Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet 2002;359:1471-7. [Crossref] [PubMed]
- Seynaeve C, Verhoog LC, van de Bosch LM, et al. Ipsilateral breast tumour recurrence in hereditary breast cancer following breast-conserving therapy. Eur J Cancer 2004;40:1150-8. [Crossref] [PubMed]
- Robson M, Svahn T, McCormick B, et al. Appropriateness of breast-conserving treatment of breast carcinoma in women with germline mutations in BRCA1 or BRCA2: a clinic-based series. Cancer 2005;103:44-51. [Crossref] [PubMed]
- Kirova YM, Stoppa-Lyonnet D, Savignoni A, et al. Risk of breast cancer recurrence and contralateral breast cancer in relation to BRCA1 and BRCA2 mutation status following breast-conserving surgery and radiotherapy. Eur J Cancer 2005;41:2304-11. [Crossref] [PubMed]
- Pierce LJ, Levin AM, Rebbeck TR, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol 2006;24:2437-43. [Crossref] [PubMed]
- Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer 2007;43:867-76. [Crossref] [PubMed]
- Garcia-Etienne CA, Barile M, Gentilini OD, et al. Breast-conserving surgery in BRCA1/2 mutation carriers: are we approaching an answer? Ann Surg Oncol 2009;16:3380-7. [Crossref] [PubMed]
- Kirova YM, Savignoni A, Sigal-Zafrani B, et al. Is the breast-conserving treatment with radiotherapy appropriate in BRCA1/2 mutation carriers? Long-term results and review of the literature. Breast Cancer Res Treat 2010;120:119-26. [Crossref] [PubMed]
- van den Broek AJ, Schmidt MK, van 't Veer LJ, et al. Prognostic Impact of Breast-Conserving Therapy Versus Mastectomy of BRCA1/2 Mutation Carriers Compared With Noncarriers in a Consecutive Series of Young Breast Cancer Patients. Ann Surg 2019;270:364-72. [Crossref] [PubMed]
- Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat 2014;144:443-55. [Crossref] [PubMed]
- Pierce LJ, Phillips KA, Griffith KA, et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat 2010;121:389-98. [Crossref] [PubMed]
- Nilsson MP, Hartman L, Kristoffersson U, et al. High risk of in-breast tumor recurrence after BRCA1/2-associated breast cancer. Breast Cancer Res Treat 2014;147:571-8. [Crossref] [PubMed]
- Molina-Montes E, Pérez-Nevot B, Pollán M, et al. Cumulative risk of second primary contralateral breast cancer in BRCA1/BRCA2 mutation carriers with a first breast cancer: A systematic review and meta-analysis. Breast 2014;23:721-42. [Crossref] [PubMed]
- Akdeniz D, Schmidt MK, Seynaeve CM, et al. Risk factors for metachronous contralateral breast cancer: A systematic review and meta-analysis. Breast 2019;44:1-14. [Crossref] [PubMed]
- van Sprundel TC, Schmidt MK, Rookus MA, et al. Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. Br J Cancer 2005;93:287-92. [Crossref] [PubMed]
- Evans DG, Ingham SL, Baildam A, et al. Contralateral mastectomy improves survival in women with BRCA1/2-associated breast cancer. Breast Cancer Res Treat 2013;140:135-42. [Crossref] [PubMed]
- Metcalfe K, Gershman S, Ghadirian P, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ 2014;348:g226. [Crossref] [PubMed]
- Heemskerk-Gerritsen BA, Rookus MA, Aalfs CM, et al. Improved overall survival after contralateral risk-reducing mastectomy in BRCA1/2 mutation carriers with a history of unilateral breast cancer: a prospective analysis. Int J Cancer 2015;136:668-77. [PubMed]
- Xiao YL, Wang K, Liu Q, et al. Risk Reduction and Survival Benefit of Risk-Reducing Salpingo-oophorectomy in Hereditary Breast Cancer: Meta-analysis and Systematic Review. Clin Breast Cancer 2019;19:e48-65. [Crossref] [PubMed]
- Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol 2013;31:3091-9. [Crossref] [PubMed]
- Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 2009;27:5887-92. [Crossref] [PubMed]
- Evron E, Ben-David AM, Goldberg H, et al. Prophylactic irradiation to the contralateral breast for BRCA mutation carriers with early-stage breast cancer. Ann Oncol 2019;30:412-7. [Crossref] [PubMed]
- Barry M, Kell MR. Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat 2011;127:15-22. [Crossref] [PubMed]
- Hamilton JG, Genoff MC, Salerno M, et al. Psychosocial factors associated with the uptake of contralateral prophylactic mastectomy among BRCA1/2 mutation noncarriers with newly diagnosed breast cancer. Breast Cancer Res Treat 2017;162:297-306. [Crossref] [PubMed]
- D'Alonzo M, Piva E, Pecchio S, et al. Satisfaction and Impact on Quality of Life of Clinical and Instrumental Surveillance and Prophylactic Surgery in BRCA-mutation Carriers. Clin Breast Cancer 2018;18:e1361-6. [Crossref] [PubMed]
Cite this article as: Ponzone R. Do all BRCA1/2 carriers with breast cancer benefit from bilateral mastectomy? Ann Breast Surg 2019;3:23.

