
Radiotherapy and breast reconstruction: a narrative review
Introduction
Radiotherapy has become a significant entity in the algorithm of breast cancer treatment. It eliminates subclinical disease, acting as an adjunct to the surgical removal of the tumor. The benefit of radiotherapy is evident in node positive patients, those with locally advanced disease and large tumor size (1). Nevertheless, there is a general inclination to a lower threshold for the delivery of radiotherapy in breast cancer patients (2). Radiotherapy is often delivered post and not pre mastectomy, thus familiarizing the term post mastectomy radiotherapy (PMRT).
Even though radiotherapy has a substantial effect on locoregional control, disease free survival and overall survival, it has profound implications on breast reconstruction (3). Like radiotherapy, breast reconstruction has slowly made its way into treatment algorithm of breast cancer. These two separate arms of breast cancer treatment, even though both separately beneficial, can be concerning when combined. Radiotherapy can have a detrimental outcome on cosmesis, infections, complications and ultimately failure of the reconstruction (4). With a more targeted radiotherapy administration and with the advancement in breast reconstruction techniques, these concerns are somehow eased but not completely resolved. The overall goal of the plastic surgeon is to achieve an acceptable reconstructive outcome without compromising the oncologic outcome of the patient.
In this review, we will tackle the topic of breast reconstruction in the setting of radiation therapy. There is no clear consensus or evidence favoring an optimal strategy for radiotherapy and reconstruction. We will look into the unique challenges that affect the reconstructive plan when radiotherapy is delivered. Multiple factors need to be addressed by the plastic surgeon before a treatment plan is formulated. Initially a thorough understanding of the impact of radiation on tissues is essential. The question of the timing of reconstruction, whether immediate or delayed, including the time between radiotherapy and reconstruction remains to be a debatable topic. The type of reconstruction to be used; autologous, prosthetic or combined; should also be considered. This also includes the number of stages of reconstruction, specifically two staged reconstruction with tissue expander first followed by the permanent reconstruction. Finally, potential complications and how to deal with them will also be discussed. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/abs-20-71).
Effect of radiotherapy on tissue
It is critical to understand the consequences of radiotherapy on tissues before going into its effects on breast reconstruction. The numerous deleterious effects of radiation on the soft tissue biology, healing process and vascularity has been extensively studied and well described. Although radiation has been central in the destruction of malignant cells, its collateral damage to the surrounding normal tissues is of high price. The effects of radiation on the skin can be categorized into acute, subacute, and chronic. Hence, it contributes to a set of postoperative morbidities across a long time span. In the acute setting it can cause burns, desquamation, erythema, hyperpigmentation and edema. Acute damage usually resolves after therapy is completed and so surgical complications from radiotherapy could be avoided by waiting for the effects to clinically subside. Chronic injury on the other hand often includes dryness, hypopigmentation and eventual fibrosis and hardening of the skin and subcutaneous tissue (5). The chronic damage, collectively known as late radiation tissue injury (LRTI) can develop months or years later and the majority of cases undergoing reconstruction following radiation therapy fall into this category. LRTI has been shown to be dose-dependent and life-long. Another long-term effect of radio therapy is injury to the microvasculature causing ischemia and necrosis. A decrease angiogenesis and collagen deposition results in a delay in wound healing (6). This is one of the challenges that PMRT poses on breast reconstruction; mainly fear of wound breakdown and failure of the reconstruction (7). Given the advances in the treatment and cure of breast cancer, patients are surviving longer, increasing the window for LRTI effects to be evident. Consequently, the studies on time frames have been extensive to determine the best evidence based practice guideline in decreasing the risks and complications of reconstruction in the irradiated patient, yet no specific timing of the reconstruction can limit the added risk once radiation is received. It is of utmost importance to understand the special set of increased morbidity risks associated with radiation.
Type of reconstruction
Deciding on the type of reconstruction entails a discussion between the patient and her reconstructive surgeon. A thorough understanding of the outcomes and possible complications should be addressed. Multiple patient and disease factors should be considered including patient anatomy, breast shape, breast size, availability of donor tissues, unilateral or bilateral mastectomies, comorbidities and planned adjuvant therapy (8). Radiation therapy by itself is an independent factor that needs to be considered. A systematic review by El-Sabawi et al. evaluated surgical outcomes following both autologous and prosthetic reconstruction in the setting of PMRT and/or adjuvant chemotherapy. It showed that there was little evidence to suggest that adjuvant chemotherapy can be related to any added risk of adverse events. However, PMRT was shown to be significantly associated with an increased incidence of adverse events (9). This effect of radiotherapy has been reproducibly shown to affect surgical outcomes whether given pre- or post-reconstruction.
Autologous reconstruction
Autologous reconstruction refers to recreation of the breast mound using the patient’s own tissues and is frequently considered the gold standard of breast reconstruction. It involves the transfer of tissues (skin, fat and occasionally muscle) from one part of the body to the breast (10). Autologous reconstruction provides well vascularized soft tissue that resembles the normal breast tissue. It can be easily shaped and provides a more natural result (11).
These flaps can be pedicled or free. The most commonly used flaps in reconstruction are the transverse rectus abdominus muscle (TRAM) flap as a pedicled flap or the deep inferior epigastric artery (DIEP) flap as a free flap (12). Pedicled flaps remain attached to their blood supply, whereas in free tissue transfer the blood supply of the flap is cut and anastomosed to vessels at the donor site. The choice of pedicled or free flap depends greatly on the surgeon’s expertise. If the plastic surgeon is well trained and able to perform microsurgical tissue transfer then this is often the better option (13). Free DIEPs spare the rectus abdominus muscle and subsequently has a fewer donor site bulges and hernias as compared to TRAM flaps. Because they are supplied by the dominant deep inferior epigastric system, they also have less fat necrosis, wound complications and hospital stays (14). Other than the abdomen, possible donor sites are from the thighs, buttocks and back. If there is a paucity of donor sites or the volume of the flap is smaller than what is needed, hybrid reconstruction combining both autologous and prosthetic reconstruction have been described (15). We attempt to shy away from these procedures and prefer to augment the flap with fat rather than place an implant. We believe that the whole concept of autologous reconstruction in the setting of PMRT is to decrease the complications of prosthetics and thus it would be counterproductive to use an implant.
In the setting of a patient requiring PMRT, it is the general concept that reconstruction should be delayed in an effort to minimize complications and not affect the treatment plan in case they occur. Nevertheless, in an effort to preserve the skin envelope or if patients refuse to remain without a breast mound for the time of radiation therapy, a two staged reconstruction with a tissue expander followed by an autologous flap or immediate one staged reconstruction with an autologous flap are valid options (16).
Autologous breast reconstruction, has been shown to be more resilient to the effects of PMRT. In a prospective multicenter cohort study at 11 centers, complications and patient-reported outcomes of 622 irradiated and 1,625 non-irradiated patients who received reconstruction were analyzed. This study concluded that autologous reconstruction yields superior patient-reported satisfaction and lower risk of complications than implant-based approaches among patients receiving PMRT (17). Similarly, a meta-analysis looked into the postoperative morbidity following breast reconstruction, the analysis showed that autologous reconstruction is associated with less morbidity and it was recommended to choose an autologous flap for reconstruction if PMRT is planned (18). Consequently, all studies point towards the fact that autologous tissues tolerate PMRT better than previously believed, leading to higher patient and surgeon satisfaction (2).
Immediate single stage autologous reconstruction
Immediate autologous breast reconstruction at the time of mastectomy followed by PMRT is becoming a new trend. The effects of radiotherapy on complications and cosmetic outcomes in immediate autologous breast reconstruction is still unclear. Radiation therapy causes atrophy and fat necrosis which leads to an unpredictable volume loss affecting the overall aesthetic outcome of the reconstruction (19). A systematic review in 2013 by Schaverien et al. compared immediate to delayed autologous breast reconstruction in the setting of PMRT; 25 articles were included in the review, the results showed that there is no significant difference in the overall complication rate between those who received PMRT and those who did not. There was however a significant higher mean prevalence of fat necrosis in the flaps that received radiotherapy (P<0.006). Due to radiotherapy changes this subgroup of patients the need of revisional surgery was higher (20). Multiple other studies report a similar finding; although it is possible to perform the autologous reconstruction before the PMRT, there is a higher risk of fat necrosis, skin contracture and hardening of the flap necessitating revisional surgery (21). A more recent study by Pont et al. looked specifically into quality of life after reconstruction using the BREAST-Q questionnaire; 230 patients were included and followed up for 23 months, there was no significant difference in the quality of life when comparing patients who received PMRT and those who did not (22). The main advantage that immediate reconstruction offers is an instant result and subsequently a higher patient satisfaction rate, better sexual well-being and quality of life, meaning that it could be considered in a select group of patients (23). In general, in our practice we prefer not to irradiate the autologous flaps. Reconstruction is often delayed until post radiation therapy.
There was a debate to whether the type of flap used affects fat necrosis after radiation therapy. A study by Garvey et al. showed that contrary to popular belief, the type of flap does not affect fat necrosis. Specifically when comparing free muscle sparing TRAM to free DIEP there seems to be no difference in fat necrosis (24).
Immediate two stage reconstruction: tissue expander followed by autologous reconstruction
In an attempt to avoid radiation injury to the flap and still maintain at the breast mound, a two staged reconstruction, otherwise known as delayed immediate autologous reconstruction (DIAR), could be performed. This method of reconstruction was described by Kronowitz et al. and allows both preservation of the native skin of the breast along with protection of the psychological well-being of the patient. Essentially it offers patients the benefit of a skin sparing mastectomy and at the same time limits the radiation induced complications of breast reconstruction (25). Nevertheless, the presence of a tissue expander is not risk free. When compared to patients who underwent delayed autologous reconstruction the DIAR group had slightly rates of infection. Outcomes related to revision surgery for better contouring, volume adjustment and symmetry indicated that the DIAR group required less secondary procedures then the delayed group (60.8 and 78.8 percent, respectively; P=0.008) (26). This result was found to be reproducible; DIAR patients had an overall superior aesthetic outcome regarding breast contour size and position (27).
It is controversial whether it is absolutely necessary to deflate the tissue expander before the delivery of radiotherapy. Historically, it was assumed that deflation gives the radiotherapist more control similar to a flat chest (28). More recent studies show that this is not essential (29). Keeping the expander inflated may limit radiation therapy induced complications such as seroma formation and capsular contraction (30).
Delayed autologous reconstruction
It may be that this reconstructive technique poses the least risk on the adjuvant treatment plan as it spares patients of the acute complications of reconstruction (21). In previously irradiated patients, autologous reconstruction is preferred (Figure 1). It is essential to introduce well vascularized tissue to the area of the breast to ensure proper wound healing (8). The downside to this technique is that the skin of the flap is used to replace the mastectomy skin thus making the overall aesthetic result less appealing (26). Nevertheless, when assessing patient reported satisfaction with the reconstruction and quality of life using the BREAST-Q questionnaire, patients with delayed breast reconstruction were shown to have similar results as those who underwent immediate two staged reconstruction (31).
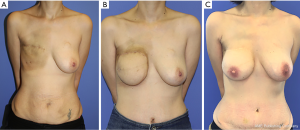
Many studies addressed the optimal timing to perform the reconstruction after radiotherapy. Radiotherapy may cause injury to the internal mammary vessels, the most commonly used recipient vessels in breast reconstruction with free tissue transfer. Since the effects of radiation therapy take time to resolve, it is recommended to allow for appropriate timing for the vessels to heal before attempting microvascular anastomosis (32). A study by Baumann et al. on 182 patients showed that waiting 12 months before the second stage is ideal as it limits the risk of microvascular thrombosis and flap loss (33). A more recent study by Mull et al. on 454 patients showed that there is no significant difference in major complications such as flap failure and total flap loss if the reconstruction was performed before or after 12 months of radiotherapy (34). Though a specific cut off time for free flap reconstruction after radiotherapy is still not clear, it is the general consensus that earlier reconstructions are more susceptible to complications and flap loss. In our practice, the earliest we perform autologous reconstruction with free tissue transfer on patients in 9 months. We feel that 6 months is too soon and have experienced difficulty with preparation of the internal mammary vessels.
Reconstruction with fat
Fat grafting has played a major controversial role in breast reconstruction from filling defects of partial mastectomies, to being an adjunct to implant based or autologous based reconstruction all the way to being the only method of reconstruction in selected cases. In patients with small breast size, especially in unilateral cases, fat grafting-only reconstruction can be utilized. A meta-analysis involving 1,011 breast reconstruction with fat grafting only showed that the number of fat grafting sessions needed to complete a breast reconstruction was significantly higher for the irradiated patients than for the nonirradiated patients. The mean volume injected per session was 230 mL, with patients requiring 2–4 sessions. There was a significantly higher complication rate in the irradiated group than in the nonirradiated group. In general, a balance is required. The more volume injected the higher the complication rates, but the less volume injected the more the number of fat grafting sessions required. Interestingly, the type of mastectomy (modified radical vs. skin sparing) was not shown to be significant in affecting the number of fat grafting sessions needed or the rate of complications (35).
Prosthetic reconstruction
Although autologous reconstruction is thought to be more cosmetically appealing and has a lower overall complication rate, a practical alternative is prosthetic reconstruction. Though it is well established in the literature that prosthetic reconstruction in the setting of PMRT has an increased risk of overall complications, infections, capsular contracture, need of revision surgery and overall failure of the reconstruction, it could be an option in a select group of patients. Patients with paucity of donor tissue, who are very thin, with no excess skin and fat, may not be candidates for pure autologous reconstruction (36). Similarly, it is an option in comorbid patients who cannot withstand the prolonged anesthesia time needed for free flap reconstruction.
Implant based reconstruction, like autologous reconstruction, can be done in a single stage or in two stages. Single stage reconstruction is mainly known as direct to implant (DTI) reconstruction. Two staged reconstruction entails a tissue expander followed by replacement with a regular implant (Figure 2). The consideration to timing, like in autologous reconstruction, is critical. The main debate with prosthetic reconstruction is weather to administer radiotherapy to the implant or to the tissue expander (37).
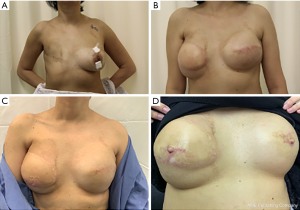
DTI reconstruction
DTI reconstruction can be appealing to patients since it provides them with a breast in a day. On the other hand, there is a general hesitation from plastic surgeons to place an implant at the time of mastectomy knowing that the patient is scheduled to receive PMRT. A recent meta analyses showed that adjuvant radiation therapy for patients who underwent immediate implant-based breast reconstruction leads to higher risks of reconstruction failure, overall complications, and capsular contracture (38). But the question that comes to mind is: is radiating implants less or more morbid then irradiating expanders? When compared to tissue expanders, DTI reconstruction had similar overall complication rates but a lower rate of failure of reconstruction (29,39). Similarly, a meta-analysis demonstrated that PMRT to implants versus to tissue expanders resulted in a significantly lower rate of reconstruction failure. But this review was based on 257 cases in three studies which is a low sample size to draw a reliable reproducible result from (40). A more recent systematic review evaluated a pooled incidence of reconstructive failure in PMRT delivered to implant compared to tissue expander showed a significantly higher failure in the tissue expander group. This review however, despite include a larger number of studies, the majority of them were single-arm studies with low level of evidence (level IV) (9).
Even though complication rates when using implants is less then tissue expanders, what these reviews failed to mention is the long-term results. Capsular contracture affecting patient quality of life and satisfaction with the cosmetic outcome is often not apparent until years after the reconstruction. A study by Jagsi et al. on 662 irradiated patients assessed the long-term patient satisfaction with the reconstruction using the BREAST-Q questionnaire showed that DTI reconstruction had the lowest patient reported satisfaction (17).
Immediate two staged prosthetic reconstruction
Like with autologous reconstruction, prosthetic reconstruction can be divided into two stages in attempt to decrease complications, preserve the skin envelope and improve patient satisfaction. There are 2 general time frames for delivery of radiotherapy; either after placement of the tissue expander or after exchange with the permanent implant. There are many studies addressing this issue with conflicting result making it unclear if it is better to deliver the PMRT before or after the implant exchange. The ideal timing of PMRT in the setting of two staged implant-based reconstruction is still unclear.
A meta-analysis by Lee et al. was conducted to compare radiating the tissue expander, meaning before the implant exchange, to radiating the permanent implant, meaning after the implant exchange. On the contrary to previous findings, no significant differences in the risks of all complications including failure of reconstruction were found between the two groups (41). Another multicentric study by Santosa et al. compared irradiation before or after implant exchange and found similar results. Outcomes were the same between the two groups with no significant predictor in complications and reconstructive failure (42). Most studies show that complication rates are similar in both groups.
The benefit that the tissue expander gives is the ability to continue inflation during adjuvant chemotherapy treatment. The expander can be filled gradually while the patient continues her treatment and then 4 weeks after completion of the treatment the exchange for a permanent implant can take place. The median time to delivery of PMRT is 8 weeks after ending chemotherapy. This means that it is feasible to perform the exchange and give the patient 4 weeks to heal without delaying her PMRT. In patients with advanced disease, who cannot wait 8 weeks for PMRT, it is inadvisable to do the exchange before radiation therapy in an attempt to prevent any delays to cancer treatment (2).
There are many important considerations worth mentioning here. The inflation status of a tissue expander at the time of PMRT can influence the development of radiation-related complications. The majority of reconstructive surgeons prefer to finalize the inflation of the expander before proceeding with PMRT, especially that the concerns regarding the effectiveness of radiation delivery on a fully inflated expander are less troubling with the new advances in radiation oncology (43).
It has been repetitively reported that delaying the exchange of the tissue expander into a permanent implant helps mitigate the risks of implant based reconstruction on a radiated breast. According to Peled et al., waiting for more than 6 months after the completion of PMRT shows a significantly lower reconstruction failure rate (44).
In an attempt to decrease complications, specifically exposure of the implant, a flap can be added in the second stage of reconstruction to help provide well vascularized tissue and subsequently protect the implant. The latissimus dorsi flap is among the most commonly used flaps in combined autologous-prosthetic reconstruction. It has acceptable perioperative and long-term morbidities and provides a stable non radiotherapized soft tissue coverage for the implant (45). If the patient is in need of skin, then the latissimus dorsi muscle is harvested with a skin paddle. But if the patient had a previous expander in place and is not in need of a skin envelope then a muscle only latissimus flap could be harvested. This can be achieved endoscopically or robotically. Because of the curvature of the back, endoscopic harvest can be technically difficult. Robotic latissimus dorsi muscle flap has been gaining popularity. It is done via a small incision that is well hidden of the axilla, complication rates are minimal, and most importantly it spares patients from having a long transverse scar (46).
In terms of cosmetic outcome, several studies have demonstrated that the results tend to be superior in the group receiving PMRT on tissue expanders compared to PMRT on implants (47). Lack of patient satisfaction and a poor cosmetic outcome can many times be due to capsular contracture. Severe capsular contracture can result in considerable patient morbidity, pain and discomfort, as well as aesthetically unpleasing result. Lee et al. showed severe capsular contracture to be remarkably lower in patients with tissue expanders compared to those with implants (41). This can be owed to the fact that aggressive capsuloplasty, whether capsulotomy or capsulectomy, is almost always performed at the second stage. A systematic review and meta-analysis that included 7 studies and 2,921 patients demonstrated increased rate of adverse events and significant reduction in patient satisfaction and cosmetic outcome within the first 5 years, post implant-based reconstruction for those patients who receive PMRT to the definitive implant (48). Hence, we find it prudent at our institution to opt for proceeding with PMRT after complete inflation of the tissue expander whenever agreed by the radiation oncologist. We then respect a time interval of at least 6 months before we exchange with the permanent implant. A pedicled autologous flap for coverage is always included. The second stage is used as an opportunity to release radiation induced contracted capsule, readjust a superiorly displaced infra-mammary fold and revise undesirable scars. We believe this protocol provides the best practice for lower risk of complications, more durable reconstruction and better cosmetic outcome and patient satisfaction.
Delayed reconstruction
Patients who have undergone previous radiation have the highest rate of failure of the reconstruction across all groups. Tissue expansion of irradiated skin can be significantly morbid, with high risk of wound breakdown and extrusion of the prosthesis, capsular contracture, implant malposition, poor cosmesis and reconstructive failure. Pure prosthetic delayed reconstruction is relatively contraindicated.
Lee et al. reviewed the impacts of pre-reconstruction radiotherapy on the surgical outcome of a delayed prosthetic breast reconstruction. This was the first meta‐analysis focusing on the risks of prior irradiation for each kind of complication of prosthetic breast reconstruction. It was based on 20 studies that included a total of 8,200 prosthetic reconstruction cases published in over a decade. This meta‐analysis was performed for eight categories of postoperative complications: infection, mastectomy flap necrosis, capsular contracture, seroma, hematoma, revision operation, reconstruction failure, and total complications. The conclusion was that the patients with prior irradiation to the breast who had implant based reconstruction had statistically and clinically significantly high risks for all complications except for hematomas. A subgroup analysis showed that this result was consistent and the same regardless of the subtype of patients according to the mastectomy type performed (49).
Acellular dermal matrix (ADM)
The use of ADM in the setting of breast reconstruction and radiation has been controversial. Despite the rational that ADMs, by providing full coverage, for tissue expanders and implants can help protect the prosthesis and hence save the reconstruction, this does not seem to hold true in the setting of radiation. In the above mentioned meta-analysis by Lee et al., a subgroup analysis comparing the pooled relative risks for complications in setting of prior radiation between groups of ADM use versus no use showed that five outcomes including infection, mastectomy flap necrosis, seroma, reconstruction failure, and total complications were increased in cases or pre-reconstruction radiation regardless of ADM use. ADM did not reduce the increased risk for complications in previously irradiated breasts compared to patients who did not receive ADM (49). Although ADM can protect adjacent soft tissue from radiation injury delivered postoperatively, its effect may not be maintained in the setting of pre‐reconstruction radiotherapy.
Nipple sparing mastectomies
With a greater trend towards nipple-areolar complex preservation in breast cancer surgeries, a systematic review and meta-analysis by Zheng et al. looked into 2 prospective and 5 retrospective studies with 3,692 patients to examine the effect of pre- or postoperative radiotherapy on the nipple areolar complex necrosis, skin flap necrosis, and local cancer recurrence in patients who underwent nipple sparing mastectomy (NSM). The review indicated that nipple areolar complex necrosis and local recurrence were the same between patients who received radiotherapy and those who did not; however, skin flap necrosis was more likely in the radiation group. This analysis however included a small number of studies with significant heterogeneity and hence it could not draw any definitive conclusion (50). The need of well-controlled trials to determine the effects of radiation in the context of NSM are still needed.
Conclusions
Breast reconstruction in the setting of PMRT is critical and must be addressed with the utmost care because of a higher incidence of complication regardless of the reconstructive technique used. A wide spectrum of choices regarding both the type of reconstruction and the method of reconstruction exist. This is why a multidisciplinary approach and collaboration with the oncologist, radiation oncologist, breast surgeon and reconstructive surgeon is essential to come up with a treatment plan tailored to the need of the patient. The common goal remains to be minimizing complications whilst maximizing satisfaction and cosmetic outcome. Because there is no level 1 evidence indication for an optimal treatment strategy, a well-informed patient about both the benefits and the involved risks is essential.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Edward I. Chang) for the series “Novel Innovations and Advancements in Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-71
Peer Review File: Available at http://dx.doi.org/10.21037/abs-20-71
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-71). The series “Novel Innovations and Advancements in Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Elsevier; 2014.
- Ho AY, Hu ZI, Mehrara BJ, et al. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol 2017;18:e742-e753. [Crossref] [PubMed]
- Yun JH, Diaz R, Orman AG. Breast reconstruction and radiation therapy. Cancer Control 2018;25:1073274818795489 [Crossref] [PubMed]
- Sekiguchi K, Kawamori J, Yamauchi H. Breast reconstruction and postmastectomy radiotherapy: complications by type and timing and other problems in radiation oncology. Breast Cancer 2017;24:511-20. [Crossref] [PubMed]
- Haubner F, Ohmann E, Pohl F, et al. Wound healing after radiation therapy: review of the literature. Radiat Oncol 2012;7:162. [Crossref] [PubMed]
- Dormand EL, Banwell PE, Goodacre TE. Radiotherapy and wound healing. Int Wound J 2005;2:112-27. [Crossref] [PubMed]
- Bentzen SM, Thames HD, Overgaard M. Latent-time estimation for late cutaneous and subcutaneous radiation reactions in a single-follow-up clinical study. Radiother Oncol 1989;15:267-74. [Crossref] [PubMed]
- Lee GK, Sheckter CC. Breast reconstruction following breast cancer Treatment—2018. JAMA 2018;320:1277-8. [Crossref] [PubMed]
- El-Sabawi B, Sosin M, Carey JN, et al. Breast reconstruction and adjuvant therapy: A systematic review of surgical outcomes. J Surg Oncol 2015;112:458-64. [Crossref] [PubMed]
- Panchal H, Matros E. Current trends in post-mastectomy breast reconstruction. Plast Reconstr Surg 2017;140:7S. [Crossref] [PubMed]
- Nahabedian MY, Patel K. Autologous flap breast reconstruction: surgical algorithm and patient selection. J Surg Oncol 2016;113:865-74. [Crossref] [PubMed]
- Macadam SA, Bovill ES, Buchel EW, et al. Evidence-based medicine: autologous breast reconstruction. Plast Reconstr Surg 2017;139:204e-229e. [Crossref] [PubMed]
- Jeong W, Lee S, Kim J. Meta-analysis of flap perfusion and donor site complications for breast reconstruction using pedicled versus free TRAM and DIEP flaps. The Breast 2018;38:45-51. [Crossref] [PubMed]
- Garvey PB, Buchel EW, Pockaj BA, et al. DIEP and pedicled TRAM flaps: a comparison of outcomes. Plast Reconstr Surg 2006;117:1711-9. [Crossref] [PubMed]
- Kanchwala S, Momeni A. Hybrid breast reconstruction—the best of both worlds. Gland Surg 2019;8:82. [Crossref] [PubMed]
- Clemens MW, Kronowitz SJ. Current perspectives on radiation therapy in autologous and prosthetic breast reconstruction. Gland Surg 2015;4:222. [PubMed]
- Jagsi R, Momoh AO, Qi J, et al. Impact of Radiotherapy on Complications and Patient-Reported Outcomes After Breast Reconstruction. J Natl Cancer Inst 2018;110:157-65. [Crossref] [PubMed]
- Barry M, Kell MR. Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat 2011;127:15-22. [Crossref] [PubMed]
- Piroth MD, Fischedick K, Wein B, et al. Fat necrosis and parenchymal scarring after breast-conserving surgery and radiotherapy with an intraoperative electron or fractionated, percutaneous boost: a retrospective comparison. Breast Cancer 2014;21:409-14. [Crossref] [PubMed]
- Schaverien MV, Macmillan RD, McCulley SJ. Is immediate autologous breast reconstruction with postoperative radiotherapy good practice?: a systematic review of the literature. J Plast Reconstr Aesthet Surg 2013;66:1637-51. [Crossref] [PubMed]
- Dewael S, Vandevoort M, Fabré G, et al. Immediate versus delayed autologous breast reconstruction: A retrospective matched cohort study of irradiated patients. J Plast Reconstr Aesthet Surg 2019;72:1769-75. [Crossref] [PubMed]
- Pont LP, Marcelli S, Robustillo M, et al. Immediate breast reconstruction with abdominal free flap and adjuvant radiotherapy: Evaluation of quality of life and outcomes. Plast Reconstr Surg 2017;140:681-90. [Crossref] [PubMed]
- Billig J, Jagsi R, Qi J, et al. Should immediate autologous breast reconstruction be considered in women who require post-mastectomy radiation therapy? A prospective analysis of outcomes. Plast Reconstr Surg 2017;139:1279. [Crossref] [PubMed]
- Garvey PB. Muscle-sparing TRAM flap does not protect breast reconstruction from postmastectomy radiation damage compared with the DIEP flap. Breast Reconstruction. Springer; 2016:1125-30.
- Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg 2004;113:1617-28. [Crossref] [PubMed]
- Patel KM, Albino F, Fan KL, et al. Microvascular autologous breast reconstruction in the context of radiation therapy: comparing two reconstructive algorithms. Plast Reconstr Surg 2013;132:251-7. [Crossref] [PubMed]
- Huis in ’t Veld EA, Long C, Sue GR, et al. Analysis of Aesthetic Outcomes and Patient Satisfaction After Delayed-Immediate Autologous Breast Reconstruction. Ann Plast Surg 2018;80:S303-S307. [PubMed]
- Motwani SB, Strom EA, Schechter NR, et al. The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:76-82. [Crossref] [PubMed]
- Lin AM, Christensen JM, Liao EC, et al. Postmastectomy Radiation Therapy on Permanent Implants or Tissue Expanders: Which is Better? Ann Surg 2019; [Crossref] [PubMed]
- Woo KJ, Paik JM, Bang SI, et al. The impact of expander inflation/deflation status during adjuvant radiotherapy on the complications of immediate two-stage breast reconstruction. Aesthetic Plast Surg 2017;41:551-9. [Crossref] [PubMed]
- Kamel GN, Nash D, Jacobson J, et al. Patient-Reported Satisfaction and Quality of Life in Postmastectomy Radiated Patients: A Comparison between Delayed and Delayed Immediate Autologous Breast Reconstruction in a Predominantly Minority Patient Population. J Reconstr Microsurg 2019;35:445-51. [Crossref] [PubMed]
- Kronowitz SJ. Delayed-immediate breast reconstruction: technical and timing considerations. Plast Reconstr Surg 2010;125:463-74. [Crossref] [PubMed]
- Baumann DP, Crosby MA, Selber JC, et al. Optimal timing of delayed free lower abdominal flap breast reconstruction after postmastectomy radiation therapy. Plast Reconstr Surg 2011;127:1100-6. [Crossref] [PubMed]
- Mull AB, Qureshi AA, Zubovic E, et al. Impact of time interval between radiation and free autologous breast reconstruction. J Reconstr Microsurg 2017;33:130-6. [Crossref] [PubMed]
- Herly M, Orholt M, Larsen A, et al. Efficacy of breast reconstruction with fat grafting: A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2018;71:1740-50. [Crossref] [PubMed]
- Nelson JA, Disa JJ. Breast reconstruction and radiation therapy: an update. Plast Reconstr Surg 2017;140:60S-68S. [Crossref] [PubMed]
- Singh P, Hoffman K, Schaverien MV, et al. Neoadjuvant radiotherapy to facilitate immediate breast reconstruction: a systematic review and current clinical trials. Ann Surg Oncol 2019;26:3312-20. [Crossref] [PubMed]
- Pu Y, Mao TC, Zhang YM, et al. The role of postmastectomy radiation therapy in patients with immediate prosthetic breast reconstruction: A meta-analysis. Medicine (Baltimore) 2018;97:e9548 [Crossref] [PubMed]
- Fuertes V, Francés M, Casarrubios JM, et al. Implant-based immediate breast reconstruction: failure rate when radiating the tissue expander or the permanent implant—a meta-analysis. Gland Surg 2020;9:209. [Crossref] [PubMed]
- Lam TC, Hsieh F, Boyages J. The effects of postmastectomy adjuvant radiotherapy on immediate two-stage prosthetic breast reconstruction: a systematic review. Plast Reconstr Surg 2013;132:511-8. [Crossref] [PubMed]
- Lee KT, Mun GH. Optimal Sequencing of Postmastectomy Radiotherapy and Two Stages of Prosthetic Reconstruction: A Meta-analysis. Ann Surg Oncol 2017;24:1262-8. [Crossref] [PubMed]
- Santosa KB, Chen X, Qi J. Postmastectomy radiation therapy and two-stage implant-based breast reconstruction: is there a better time to irradiate? Plast Reconstr Surg 2016;138:761-9. [Crossref] [PubMed]
- Chen SA, Hiley C, Nickleach D, et al. Breast reconstruction and post-mastectomy radiation practice. Radiat Oncol 2013;8:45. [Crossref] [PubMed]
- Peled AW, Foster RD, Esserman LJ, et al. Increasing the time to expander-implant exchange after postmastectomy radiation therapy reduces expander-implant failure. Plast Reconstr Surg 2012;130:503-9. [Crossref] [PubMed]
- Sood R, Easow JM, Konopka G, et al. Latissimus dorsi flap in breast reconstruction: recent innovations in the workhorse flap. Cancer Control 2018;25:1073274817744638 [Crossref] [PubMed]
- Selber JC, Baumann DP, Holsinger FC. Robotic latissimus dorsi muscle harvest: a case series. Plast Reconstr Surg 2012;129:1305-12. [Crossref] [PubMed]
- Cordeiro PG, Albornoz CR, McCormick B, et al. What Is the Optimum Timing of Postmastectomy Radiotherapy in Two-Stage Prosthetic Reconstruction: Radiation to the Tissue Expander or Permanent Implant? Plast Reconstr Surg 2015;135:1509-17. [Crossref] [PubMed]
- Magill LJ, Robertson FP, Jell G, et al. Determining the outcomes of post-mastectomy radiation therapy delivered to the definitive implant in patients undergoing one-and two-stage implant-based breast reconstruction: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2017;70:1329-35. [Crossref] [PubMed]
- Lee KT, Mun GH. Prosthetic breast reconstruction in previously irradiated breasts: A meta-analysis. J Surg Oncol 2015;112:468-75. [Crossref] [PubMed]
- Zheng Y, Zhong M, Ni C, et al. Radiotherapy and nipple-areolar complex necrosis after nipple-sparing mastectomy: a systematic review and meta-analysis. Radiol Med 2017;122:171-8. [Crossref] [PubMed]
Cite this article as: Karami RA, Ghanem OA, Ibrahim AE. Radiotherapy and breast reconstruction: a narrative review. Ann Breast Surg 2020;4:17.

