
Latissimus dorsi flap: safe, reliable and versatile workhorse flap in the era of minimally invasive breast surgery—a case series
Introduction
Latissimus dorsi (LD) flap is a versatile and reliable autologous flap reconstruction for a variety of breast defects with acceptable perioperative and long-term morbidities (1). Recent innovations to the traditional LD flap, including the extended LD flap, ‘scar-less’ approach, mini-LD and the muscle-sparing LD further increase the use of this flap to suit different patient and disease characteristics (2). However, the application of endoscopic LD flap and its variations in minimally invasive breast surgery has not been previously described. In this article, we describe our early experience of endoscopically harvested LD flap as part of the minimally invasive breast reconstruction armamentarium. To the best of our knowledge, there has been no reported series on multiple variations of endoscopically-harvested LD flap as a reconstructive option after minimally invasive oncological resection. Herein, we present the following article in accordance with the AME Case Series reporting checklist (available at http://dx.doi.org/10.21037/abs-20-62).
Methodology
Patient selection and outcome measures
Consecutive patients who underwent breast reconstruction using LD flap between January to March 2020 in a tertiary hospital with a full-fledged one stop breast center managing the whole spectrum of benign to complex malignant breast conditions, were included in this case series and analyzed. All patients had pre-operative ultrasound breast, mammography and/or magnetic resonance imaging to assess the extent of cancer in order to decide on the type of oncological resection. Contraindication to LD flap harvesting was previous thoracotomy or surgery which would compromise the blood supply to the LD muscle (2).
Data collection included clinicopathological characteristics of tumor, types of surgery and reconstruction, operative time, blood loss, length of hospital stay, surgical outcomes and complications. Oncological outcomes were not assessed due to the short follow up duration. Aesthetic outcomes evaluations with clinical and post-operative photography-based assessments were performed, in which patients’ consent was obtained. Patients and surgeons were asked to grade their satisfaction from a scale of 5: 1- extremely poor, 2- poor, 3- fair, 4- good, 5-excellent. Exemption was obtained from authors’ Institution Review Board (IRB) as no identifiable patients’ information was required in this study.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Surgical techniques
We present five consecutive cases with three different variations of LD flap in different scenarios using minimally invasive techniques, for both oncological resection and reconstruction. The different surgical techniques and their applications are discussed as follows:
Variation 1: Endoscopic skin-sparing mini LD flap (Figure 1)
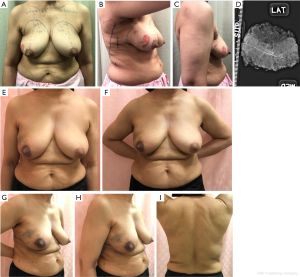
The skin sparing mini-LD is suitable for partial breast reconstruction where the breast defect is more than 20% of the breast volume. The use of endoscopic dissection allows for the flap harvest to be performed via a small hidden incision placed along the anterior axillary line. Disadvantages include longer operative time (when compared to a level I oncoplastic procedure) and complications associated with LD flap harvest.
Case 1—Right breast wide excision with immediate endoscopic skin sparing mini LD flap reconstruction
Despite extensive pleomorphic microcalcifications distributed over the upper outer quadrant, the patient was keen for breast conserving surgery (BCS). LD bulk requirement was estimated to be small and hence a skin-sparing mini LD flap reconstruction would suffice. Flap harvest was performed endoscopically through a 3 cm incision over the anterior axillary line while the wide excision and flap insertion were achieved via a 2 cm peri-areolar incision.
Variation 2: Endoscopic skin-sparing full LD flap (Figure 2)
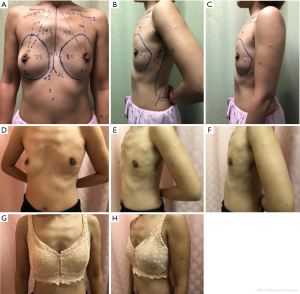
Skin-sparing full LD flap may be used for full breast reconstruction in ladies with small to moderately sized breast. In larger sized breast, it may be combined with an implant to achieve the desired volume. The use of endoscopic dissection allows for better visualization and ease of dissection while minimizing the size of the incision (located either in the axilla or along the anterior axillary line), thus avoiding an unsightly donor site incision.
Case 2—Endoscopic right nipple sparing mastectomy and endoscopic skin sparing full LD flap reconstruction for right breast cancer
This was a 49 years old lady with a 2 cm invasive carcinoma in an A-cup breast. BCS would result in significant deformity. Clinical pinch test assessed that a skin-sparing full LD flap to be adequate for full breast reconstruction. She underwent a nipple sparing mastectomy with LD flap reconstruction both performed endoscopically through a single 3 cm anterior axillary line incision.
Case 3—Endoscopic left nipple sparing mastectomy with endoscopic skin sparing full LD flap reconstruction for borderline phyllodes tumor
This patient presented with a large 7 cm × 4 cm borderline phyllodes tumor which was removed endoscopically. The margins were inadequate but with an A-cup breast, further margin excision would result in significant deformity. She underwent a nipple sparing mastectomy with skin sparing LD flap reconstruction completely performed endoscopically via a single 2.5 cm anterior axillary line incision.
Variation 3: Hybrid endoscopic full LD myocutaneous flap (Figure 3)
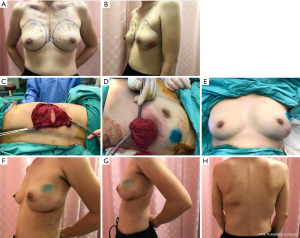
In the full LD myocutaneous flap, the inclusion of the skin paddle and subcutaneous fat allows for a larger bulk when compared to skin-sparing LD flap. The hybrid endoscopic technique uses endoscopic-assisted technique for better visualization and accurate dissection, thereby requiring a shorter donor site scar. This technique is useful for either partial or full breast reconstruction depending on the required size/volume of LD flap.
Case 4—Left breast wide excision with hybrid endoscopic full LD myocutaneous flap reconstruction
Vacuum-assisted biopsy of a 1 cm lower outer quadrant left breast lump revealed invasive ductal carcinoma. Pre-operative breast MRI demonstrated significant enhancement of 3 cm around the cavity, suspicious for residual disease. The patient was slim built (BMI 16 kg/m2) but with a C-cup breast. She had extremely little adiposity for local advancement or perforator flaps and her LD bulk was assessed to be small. The BCS was performed with an inframammary incision. To achieve adequate volume to replace the BCS cavity, a full LD flap including skin paddle was harvested with a 4 cm × 2 cm elliptical skin paddle. The flap was initially raised from the skin paddle to the anatomical boundaries with lighted retractors. Subsequently, endoscopic dissection of the posterior and inferior portion of the LD flap allowed the dissection to reach the anterior superior iliac spine with ease.
Case 5—Endoscopic right skin sparing mastectomy with hybrid endoscopic full LD myocutaneous flap reconstruction
This 69-year-old patient with a C-cup breast, had a retroareolar invasive cancer. A full LD myocutaneous flap was required to obtain enough volume for a full breast reconstruction after a mastectomy. The mastectomy was performed via a periareolar incision. The full LD flap was harvested with an 8 cm × 3 cm elliptical skin paddle, initially raised from the skin paddle to the anatomical boundaries with lighted retractors and subsequently, the posterior and inferior portion was raised endoscopically.
Findings and results
The mean age of patients was 56 (range, 45–69) years old. All had early non-metastatic breast cancer, no evidence of skin or chest wall invasion and no clinical evidence of lymph node metastases. Only 1 was overweight (BMI >23.1 kg/m2). Most had small to moderate sized breast and an adequate LD bulk. Two patients who underwent mastectomy had A-cup breasts, whereas three other patients had B to C-cup breasts.
Mean pathological tumor size was 2.8 cm (range, 8 mm to 7 cm). Two patients had multifocal tumor. All had invasive carcinoma except one with borderline phyllodes. Of the former, the final histological stages were Stage 1 (2 cases) and Stage 2A (2 cases). Case 3 had 1.2 mm of residual borderline phyllodes in the mastectomy specimen. Sentinel lymph node biopsy performed for the 4 cases of invasive carcinoma and were negative. Intra-operative frozen section analysis of sub-nipple biopsies performed for nipple sparing mastectomy was negative. All had adequate margin. Specimen weight ranged from 34 to 51 g for wide excision cases and 117 to 309 g for mastectomy specimens (Table 1).
Table 1
| Case No. | Baseline demographics | Tumor characteristics | Surgery | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Co-morbidities | BMI | Cup Size | Location | Tumor size (cm) | Histology | Resection | Axillary procedure | Reconstruction | |||
| 1 | 65 | Nil | 22 | B | Right upper outer quadrant | 2 clusters of microcalcifications 2 cm apart | Invasive mucinous carcinoma | Wide excision | SLNB | Endoscopic skin-sparing mini LD flap | ||
| 2 | 49 | Nil | 21 | A | Left lower outer quadrant | 2.0 × 2.0 × 1.2 | Invasive ductal carcinoma | Nipple sparing mastectomy | SLNB | Endoscopic skin-sparing full LD flap | ||
| 3 | 45 | Ex-smoker | 21.5 | A | Right retroareolar | 7.2 × 2.9 × 4.2 | Borderline phyllodes tumour | Nipple sparing mastectomy | Nil | Endoscopic skin-sparing full LD flap | ||
| 4 | 51 | Nil | 16.8 | C | Location | 1.0 × 0.9 × 0.6 | Invasive ductal carcinoma | Wide excision | SLNB | Hybrid endoscopic full LD myocutaneous flap | ||
| 5 | 69 | Nil | 24 | C | Right upper outer quadrant | 2.8 × 2.0 × 1.9 | Invasive ductal carcinoma | Skin sparing mastectomy | SLNB | Hybrid endoscopic full LD myocutaneous flap | ||
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor 2; SLNB, sentinel lymph node biopsy; LD, latissimus dorsi.
The mean overall operation, resection and reconstruction time was 339 (range, 265–425), 126 (range, 50–180) and 217 minutes (range, 200–230) respectively. The mean blood loss for all was less than 15 ml and mean hospitalization was 1 day.
All patients’ wounds healed well except for Case 5, who developed minimal skin flap necrosis without infection, at the peri-areolar incision. It healed spontaneously within a week, without the need for dressing or debridement. Two patients received their adjuvant therapy within a month from surgery. The two cases who had hybrid endoscopic full LD myocutaneous flap reconstruction commenced adjuvant therapy 5 weeks post-surgery. Case 4’s adjuvant radiotherapy was delayed due to eczematous flare while Case 5, the delay was due to skin flap necrosis. Nonetheless, this was still within the acceptable duration of 6–8 weeks (3,4) where survival outcome would not be affected.
Aesthetic outcome assessed by the performing surgeon and patients graded it to be between good and excellent. All patients were satisfied with their scar appearance, wound length and location (Table 2).
Table 2
| Case No. | Op | Specimen weight (g) | Overall operating time (min)* | Oncological resection time (min) | Reconstruction time (min)@ | Blood loss (mL) | Hospital stay (days) | Margin involvement | Complications | Time to adjuvant therapy(weeks)^^ | Patients’ satisfaction | Surgeon’s satisfaction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | WE | 51 | 335 | 95 | 215 | <15 | 1 | No | No | 3.5 | Excellent | Excellent |
| 2 | NSM | 117 | 425 | 180 | 225 | <15 | 1 | No | No | 4 | Good | Good |
| 3 | NSM | 142 | 270 | 150 | 215 | <15 | 1 | No | No | Nil adjuvant | Excellent | Excellent |
| 4 | WE | 34 | 265 | 50 | 200 | <15 | 1 | No | No | 5 | Excellent | Excellent |
| 5 | SSM | 309 | 400 | 155 | 230 | <15 | 1 | No | Skin flap necrosis # | 5 | Excellent | Good |
*, including positioning and preparation time; @, reconstruction time is inclusive of LD flap harvest and subsequent flap inset duration; ^^, time to adjuvant therapy refers to duration from date of surgery to start of adjuvant therapy; #, mild skin flap necrosis around peri-areolar incision which healed spontaneously within a week without evidence of infection and the need for dressing or debridement. WE, wide excision; NSM, nipple-sparing mastectomy; SSM, skin-sparing mastectomy; RT, radiotherapy; Chemo, chemotherapy; Endocrine, endocrine therapy.
Discussion
Breast reconstruction in Asian women have been reported to be different from their Western counterparts (5,6) due to smaller, non-ptotic breasts common to Asian ladies. To further compound the problem, there is a possibility of having a well-endowed but extremely slim lady such as Case 4 reported in our series which further limits the choices for autologous reconstruction.
Despite numerous studies (5-7) on reconstruction options for Asian women, due to their unique physical attributes, no clear advantage have been subscribed to any one technique. Implant-based reconstruction is not ideal in thin women owing to implant visibility and palpability. Moreover, due to recent reports of BIA-ALCL (8), smooth implants are preferred over textured anatomical implants, which leads to poor aesthetic outcome in thin ladies. The lack of abdominal and lateral chest adiposity in Asian women limits the use of autologous local oncoplastic procedures or TRAM or DIEP flap.
LD flap reconstruction, on the other hand, provides adequate bulk for reconstruction in thin ladies without disadvantages of other options. It is suitable for partial or full breast reconstruction (2). The main drawback with conventional LD flap is the long and unsightly donor scar. To overcome this, the LD flap can be safely harvested with the use of minimally invasive techniques as demonstrated in this series. In fact, some patients had the same incision for both oncological breast resection and flap reconstruction. Depending on the volume of LD required, modifications in technique via the 3 variations described in our series, can be tailored to different clinical needs.
In terms of operative safety and outcomes in our series, blood loss was minimal, all patients were discharged the next day and there was only one minor wound necrosis. This is comparable to other series of endoscopically harvested LD flap for reconstruction (9,10). The aesthetic outcomes were either good or excellent as assessed by the patient or primary surgeon using clinical and photography-based assessments.
Previous studies have separately described minimally invasive techniques for oncological resection (11) and endoscopic-assisted LD flap harvest (12,13). Our series describes for the first time, the combination of using minimally invasive oncological breast resection for breast cancer with different variations of LD flap harvested endoscopically. The limitations of this series are the small numbers and the lack of an objective assessment of aesthetic outcomes. Nonetheless, it demonstrates the technical safety and feasibility in suitable patients.
Conclusions
Our initial experience demonstrated the safety and versatility of endoscopically harvested LD flap in combination with minimally invasive breast surgery. Tailoring the appropriate LD volume harvested to suitable patients can result in excellent aesthetic outcomes. A longer follow up study will be essential to ascertain the long-term outcomes of this versatile technique.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-62
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-62). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee KT, Mun GH. A systematic review of functional donor-site morbidity after latissimus dorsi muscle transfer. Plast Reconstr Surg 2014;134:303-4. [Crossref] [PubMed]
- Sood R, Easow JM, Konopka G, et al. Latissimus Dorsi Flap in Breast Reconstruction: Recent Innovations in the Workhorse Flap. Cancer Control 2018;25:1073274817744638 [Crossref] [PubMed]
- Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, et al. Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA Oncol 2016;2:322-9. [Crossref] [PubMed]
- Gagliato DM, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 2014;32:735-44. [Crossref] [PubMed]
- Kim EK, Eom JS, Hwang CH, et al. Immediate transverse rectus abdominis musculocutaneous (TRAM) flap breast reconstruction in underweight Asian patients. Breast Cancer 2014;21:693-7. [Crossref] [PubMed]
- Engel H, Huang JJ, Lin CY, et al. Subcutaneous tissue expansion and subsequent subpectoral implantation for breast reconstruction in Asian patients: safety and outcome. Ann Plast Surg 2013;70:135-43. [Crossref] [PubMed]
- Lee JS, Kim JS, Lee JH, et al. Prepectoral breast reconstruction with complete implant coverage using double-crossed acellular dermal matrixs. Gland Surg 2019;8:748-57. [Crossref] [PubMed]
- Ghosh T, Duncavage E, Mehta-Shah N, et al. A Cautionary Tale and Update on Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J 2020;40:1288-300. [Crossref] [PubMed]
- Yang CE, Roh TS, Yun IS, et al. Immediate partial breast reconstruction with endoscopic latissimus dorsi muscle flap harvest. Arch Plast Surg 2014;41:513-9. [Crossref] [PubMed]
- Pomel C, Missana MC, Atallah D, et al. Endoscopic muscular latissimus dorsi flap harvesting for immediate breast reconstruction after skin sparing mastectomy. Eur J Surg Oncol 2003;29:127-31. [Crossref] [PubMed]
- Mok CW, Lai HW. Endoscopic-assisted surgery in the management of breast cancer: 20 years review of trend, techniques and outcomes. Breast 2019;46:144-56. [Crossref] [PubMed]
- Tan O, Aydin OE, Cinal H, et al. Latissimus dorsi flap harvest with a short incision. Microsurgery 2013;33:203-6. [Crossref] [PubMed]
- Missana MC, Pomel C. Endoscopic latissimus dorsi flap harvesting. Am J Surg 2007;194:164-9. [Crossref] [PubMed]
Cite this article as: Mok CW, Hing JXJ, Tan SM. Latissimus dorsi flap: safe, reliable and versatile workhorse flap in the era of minimally invasive breast surgery—a case series. Ann Breast Surg 2020;4:30.

