Idiopathic granulomatous mastitis
Introduction
Idiopathic granulomatous mastitis (IGM) is a benign inflammatory breast condition first described in the literature in 1972 (1). Also referred to as idiopathic granulomatous lobular mastitis, it is a rare and chronic condition characterized by relapsing sterile, draining breast abscesses. IGM is challenging clinically, as it mimics the presentation of a bacterial abscess or breast cancer. Often, the diagnosis of IGM is given when all other differential diagnoses are excluded. IGM usually presents in premenopausal women who were recently pregnant or lactating (2-4). It can occur in any race but does have a higher incidence in Hispanics (3,5-7) and people of Mediterranean descent (8).
Despite being present in the literature for the last fifty years, many things are still unknown about this complex, inflammatory disease. First, the etiology remains unclear despite many research studies investigating this disease. Many hypotheses exist for the exact mechanism of action, but an amplified autoimmune response is considered one of the top theories (1,9). Second, the gold-standard treatment modality for this disease remains highly debated within the literature with no clear consensus on the recommended treatment. The recommended treatments range from observation, steroids, methotrexate, surgical excision, and unique combinations of these treatment approaches (10). In this review, we aim to provide a clear overview of IGM as well as analyze the common treatment modalities highlighted in the literature.
Methods
A PubMed search was used to identify all articles regarding the diagnosis and treatment of IGM. A total of 616 articles from the time frame of 1972 to June 2020 were identified utilizing the search term of “granulomatous mastitis”. Each article was analyzed for the main finding of the study as well as the recurrence rate. Few articles were prospective randomized trials and many were retrospective analyses or case studies identifying the success of various treatment regimens. All pertinent articles are included online: https://cdn.amegroups.cn/static/public/ABS-2020-BBD-03-supplementary.xlsx. Institutional review board approval was not obtained since only published studies were analyzed for this review article.
A summarized version of the appendix is included in this paper (Table 1) which highlights the type of study performed, number of subjects included, which treatment modalities were compared, recurrence rates as well as findings and recommendations. Table 1 was created from the appendix by first identifying the different treatment modalities and then identifying how many published articles, in the conclusion of the manuscript, supported that treatment modality. Manuscripts supporting the various treatments were grouped together. Treatment groups that contained less than ten publications had every article included in the table. If a treatment group had more than ten published studies, then the articles were selectively chosen to be added to Table 1 if they (I) had a sample size larger than 20 subjects and (II) contributed to the field through multiple citations, robust data, supported conclusions and low recurrence rates. With the presented conflicting arguments of each treatment modality highlighted, this review will give treatment guidelines for clinicians as well as a simplified management algorithm.
Table 1
| Type of treatment | Authors | Journal | Year | Total number of subjects | Type of study | Recurrence rate (n, %) | Treatment modalities compared | Findings and recommendations |
|---|---|---|---|---|---|---|---|---|
| Observation | Pandey et al. (5) | The Breast | 2014 | 49 | Prospective case series | 0 (0.0%) | Compared patients with IGM to observation, surgery, and steroid therapy | Recommended observational approach for painless cases; for painful cases, recommended against surgical treatment, favoring steroids |
| Ma et al. (11) | Breast Care | 2020 | 970 | Systemic review and meta-analysis | N/A | Reviewed 21 studies that compared surgical excision, steroids, abscess drainage, antibiotics, and observation | Concluded that observation for early IGM patients is beneficial | |
| Bouton et al. (12) | The American J of Surg | 2015 | 37 | Retrospective chart review | 3 (11.1%) | Compared antibiotic treatment, drainage, excision, and observation | Proposed that IGM is a self-limited condition which will resolve spontaneously without treatment | |
| Mahlab-Guri et al. (13) | IMAJ | 2015 | 17 | Case series | 1 (25.0%) | Compared oral steroids to oral steroids plus MTX and excision | The recommended treatment of choice is observation; corticosteroids recommended in severe cases | |
| Hur et al. (14) | JKSS | 2013 | 50 | Retrospective chart review | 1 (12.5%) | Five treatment groups: observation, antibiotics, steroid, drainage, and surgical excision | Concluded that observation is recommended when lesions were small and symptoms were mild | |
| Davis et al. (15) | Surgery | 2019 | 120 | Retrospective chart review | 19 (16.6%) | Analyzed observation only | Recommended observation; with resolution occurring at an average of 5 months | |
| Surgery | Yabanoglu et al. (16) | The Breast | 2015 | 77 | Comparative study | 0 (0.0%) | Compared steroids and surgical excision | Wide surgical excision was the preferred approach for treating patients with IGM because of the low recurrence rate |
| Alrayes et al. (17) | The Breast | 2019 | 29 | Retrospective chart review | 0 (0.0%) | Surgical excision only | Authors concluded that surgical excision is recommended for the treatment of IGM | |
| Korkut et al. (18) | Eurasian J Med | 2015 | 73 | Retrospective chart review | 4 (11.1%) | Compared incision and drainage, surgical excision, and corticosteroids | Surgical excision is recommended for IGM resolution; corticosteroids recommended in select patients | |
| Hur et al. (14) | JKSS | 2013 | 50 | Retrospective chart review | 1 (8.3%) | Compared five treatment method groups: observation, antibiotics, steroid, drainage, and surgical excision | Concluded that surgery was best treatment modality when a lesion was determined to be mass forming or a localized abscess due to fast recovery and high success rate | |
| Erozgen et al. (19) | Breast Cancer Res Treat | 2010 | 25 | Retrospective chart review | N/A | Compared oral steroids and surgical excision | Authors proposed that surgical treatment is the first line treatment due to corticosteroid therapy having extensive complications | |
| Kok et al. (20) | Surgeon | 2010 | 43 | Retrospective cohort study | 10 (25%) | Compared surgical excision and corticosteroids | Recommended treatment with complete surgical excision and drainage as first-line therapy | |
| Yau et al. (21) | Ann Plast Surg | 2010 | 31 | Retrospective chart review | 1 (3.2%) | Compared surgical excision and antibiotics | Concluded surgical intervention as an effective method for treating IGM | |
| Oral steroids | Deng et al. (22) | J of Clin Pathology | 2017 | 65 | Retrospective case report | 12 (18.5%) | Oral corticosteroids only | Authors concluded that an effective treatment option is corticosteroids after removal of the lesion using the Mammotome biopsy system |
| Cetin et al. (23) | World J Surg | 2019 | 124 | RCT | 20.70% | Compared the different steroid administration modalities of topical, systemic, and topical plus systemic | Concluded that oral, systemic steroids are 80% effective for complete response with 20% recurrence rate | |
| Mahmodlou et al. (24) | Electron Physician | 2017 | 48 | Retrospective cohort study | 3 (6.25%) | Oral corticosteroids only | Authors concluded that steroid therapy is an effective treatment modality for IGM | |
| Aghajanzadeh et al. (25) | The Breast | 2015 | 206 | Retrospective chart review | 11 (5.5%) | Compared the treatments of surgical excision, oral steroids, steroids plus methotrexate, steroids plus bromocriptine, and surgery plus steroids plus antibiotics | Recommended oral corticosteroids as the first line of treatment | |
| Pandey et al. (5) | The Breast | 2014 | 49 | Observational prospective cohort study | 9 (20.5%) | Compared subjects with IGM to observation, surgery, and steroid therapy | Authors recommended steroid therapy as an effective non-surgical option | |
| Montazer et al. (26) | Asian Pac J Cancer Prev, | 2020 | 30 | RCT | N/A | Contrasted high and low dose corticosteroids | Concluded that high dose prednisolone is more beneficial than low dose prednisolone due to having a higher success rate with lower recurrence; could reduce the need for surgery | |
| Mizrakli et al. (27) | Surg Today | 2015 | 49 | Retrospective chart review | N/A | Compared oral corticosteroids, antibiotic therapy, and surgical excision | Authors concluded that systemic corticosteroids are an appropriate treatment option for IGM | |
| Shin et al. (28) | BMC Women’s Health | 2017 | 34 | Retrospective chart review | 5 (25.0%) | Compared wide excision, corticosteroids, and abscess drainage | Recommended first line treatment should be steroid therapy with or without abscess drainage | |
| Oral steroids + surgical management | Wang et al. (29) | J of Invest Surg | 2019 | 200 | Retrospective chart review | 8 (5.1%) | Compared steroid therapy alone to surgical excision after steroid therapy | Authors concluded that surgery after steroid therapy is a more satisfactory treatment for IGM than steroid therapy alone |
| Akcan et al. (30) | Breast Care | 2014 | 74 | Retrospective chart review | 0 (0.0%) | Analyzed surgical excision with or without oral corticosteroids | Recommended systemic steroid therapy with surgical resection as first-line treatment | |
| Oran et al. (31) | The Breast | 2013 | 46 | Retrospective chart review | 0 (0.0%) | Compared surgical excision, steroid therapy, and steroid therapy plus surgical excision | Authors concluded that surgical excision plus steroid therapy is first line management for recurrence | |
| Gurleyik et al. (32) | J Breast Cancer | 2012 | 19 | Retrospective chart review | 1 (5.2%) | Compared oral corticosteroids and consecutive surgical excision after follow-up | Recommended the first line treatment of IGM is corticosteroids followed by consecutive surgical excision of the remaining lesions | |
| Karanlik et al. (33) | Breast Care (Basel) | 2014 | 60 | Prospective, non-randomized observational study | 0 (0.0%) | Compared low-dose oral corticosteroid therapy alone to low-dose corticosteroid therapy followed by surgery | Concluded that surgical excision after steroid therapy is more advantageous treatment option than steroid therapy alone | |
| Oral steroids + methotrexate | Sheybani et al. (34) | The American College of Obstetricians and Gynecologists | 2015 | 22 | Prospective cohort study | N/A | Compared oral steroids to oral steroids plus methotrexate | Concluded the treatment of choice is corticosteroids and methotrexate, with or without surgery |
| Aghajanzadeh et al. (25) | The Breast | 2015 | 206 | Retrospective chart review | 16 (28.6%) | Compared the treatments of surgical excision, oral steroids, steroids plus methotrexate, steroids plus bromocriptine, and surgery plus steroids and antibiotics | Authors recommended oral steroids plus methotrexate when patient is prone to a recurrence | |
| Kim et al. (35) | ANZ J. Surg | 2003 | 5 | Case reports | 0 (0.0%) | Analyzed methotrexate and corticosteroid usage in surgical-resistant cases | Recommended a low weekly oral dose of methotrexate plus corticosteroids in surgical-resistant cases | |
| Topical steroids | Altintoprak et al. (36) | World Journal of Surgery | 2015 | 47 | Retrospective chart review | 3 (10.7%) | Analyzed topical steroids only | Concluded that topical steroids are a recommended treatment modality for IGM characterized by skin changes and the benefit of no steroid side effects |
| Altintoprak et al. (37) | Korean J Intern Med | 2011 | 1 | Case report | 0 (0.0%) | Analyzed topical steroid usage with a low dose oral steroid | Authors recommend topical steroids for IGM management | |
| Cetin et al. (23) | World J Surg | 2019 | 124 | RCT | 5 (14.7%) | Compared the different steroid administration modalities of topical, systemic, and topical plus systemic | Concluded that topical steroids are first line treatment due to the decrease in side effects | |
| Gunduz et al. (38) | The Breast | 2014 | 11 | Case report | 2 (18.2%) | Analyzed topical steroids only | Authors concluded topical steroids are an effective therapeutic approach | |
| Steroid injections | Tang et al. (39) | Journal of Surgical Research | 2020 | 49 | Retrospective cohort study | 0 (0.0%) | Compared the treatment methods of observation, steroid injection, and surgical resection | Authors concluded that intralesional steroid injection is an effective treatment option |
| Kim et al. (40) | J of Surgical Ultrasound | 2016 | 15 | Retrospective cohort study | 0 (0.0%) | Authors compared steroid injections with or without oral steroid administration | Intralesional steroid injection of Triamcinolone is an effective treatment modality for IGM | |
| Alper et al. (41) | The Breast | 2020 | 28 | Prospective cohort study | 0 (0.0%) | Analyzed steroid injections only | Injection of steroids for the treatment of IGM is an effective method with minimal complications | |
| Munot et al. (42) | European Journal of Surgical Oncology | 2012 | 4 | Case reports | 0 (0.0%) | Analyzed steroid injections only | Authors recommend injection of steroids into the breast cavity; all patients had a complete clinical and radiological response with no side effects | |
| Methotrexate and other agents | Raj et al. (43) | Rheumatology | 2004 | 3 | Case reports | N/A | Compared the treatment modalities of methotrexate plus corticosteroids and methotrexate plus azathioprine | Recommended the use of methotrexate with azathioprine without long-term steroids use to avoid steroid side effects |
| Wang et al. (44) | The Breast | 2019 | 1 | Case reports | 0 (0%) | Investigated the treatment method of methotrexate plus etanercept | IGM was successfully treated with etanercept combined with methotrexate | |
| Schmajuk et al. (45) | J Rheumato | 2009 | 2 | Case reports | 0 (0%) | Analyzed the treatment of methotrexate alone | Authors recommend treatment utilizing methotrexate as a solo therapy | |
| Farouk et al. (46) | World J Surg | 2017 | 30 | Prospective case report | 0 (0%) | Examined Rifampicin as a solo medical therapy for IGM | Concluded that Rifampicin is an effective solo medical therapy for IGM, can be used as an alternative to corticosteroids and surgery | |
| Akbulut et al. (47) | Breast J. | 2011 | 108 | Case reports | 2 (12.5%) | Investigated methotrexate plus corticosteroids as a treatment regimen | Methotrexate can prevent complications in IGM cases without steroid side effects | |
| Steuer et al. (48) | JAMA Dermatology | 2020 | 32 | Case reports | N/A | Compared antibiotics and antibiotics plus methotrexate | Authors concluded first line IGM treatment is doxycycline twice daily followed by the second line treatment being methotrexate | |
| Haddad et al. (49) | The Breast | 2020 | 17 | Retrospective cohort study | 3 (17.6%) | Analyzed methotrexate only | MTX monotherapy is beneficial for the treatment of IGM | |
| Postolova et al. (50) | J Rheumatol | 2020 | 19 | Case reports | 3 (15.8%) | Analyzed the usage of methotrexate after failed treatment with antibiotics, prednisone, and surgical intervention | Authors recommend methotrexate for patients who failed treatment with antibiotics, prednisone, and surgical intervention | |
| Comparative studies | Azizi et al. (51) | The Breast | 2020 | 474 | Retrospective cohort study | N/A | Compared patients in four groups: medical treatment only, surgical treatment only, a combination of medical and surgical treatment, and no treatment (self-resolving) | Concluded that the most common treatment was medical therapy |
| Wang et al. (29) | J of Invest Surg | 2019 | 200 | Retrospective chart review | N/A | Compared surgery after steroid therapy and steroid therapy alone | Authors concluded that surgery after steroid therapy is a more satisfactory treatment for IGM than steroid therapy alone | |
| Ma et al. (11) | Breast Care | 2020 | 970 | Systemic review and meta-analysis | N/A | Reviewed 21 studies that compared surgical excision, steroids, abscess drainage, antibiotics, and observation | Recommended observation for early IGM patients | |
| Steuer et al. (48) | JAMA Dermatology | 2020 | 32 | Case reports | N/A | Analyzed antibiotics and methotrexate | Authors concluded first line IGM treatment is doxycycline twice daily followed by the second line treatment being methotrexate | |
| Cetin et al. (23) | World J Surg | 2019 | 124 | RCT | N/A | Compared different steroid administration modalities of topical, systemic, and topical plus systemic | Concluded that topical steroids are first line treatment due to the decreased side effects | |
| Atak et al. (52) | Breast Dis. | 2015 | 40 | Retrospective chart review | N/A | Authors compared the treatment methods of antibiotics and anti-inflammatory agents, steroids, abscess drainage, and surgical excision | Concluded that surgical excision is the best treatment modality for IGM | |
| Akcan et al. (30) | Breast Care | 2014 | 74 | Retrospective chart review | N/A | Compared surgical excision with or without oral corticosteroids | Authors recommend systemic steroid therapy with surgical resection as first-line treatment | |
| Oran et al. (31) | The Breast | 2013 | 46 | Retrospective chart review | N/A | Three different treatment modalities were examined: surgical excision, steroid therapy, and steroid therapy plus surgical excision | Authors concluded that surgical excision or steroid therapy is first line | |
| Hur et al. (14) | JKSS | 2013 | 50 | Retrospective chart review | N/A | Five treatment method groups: observation, antibiotics, steroid, drainage, and surgical excision | Concluded that surgery be best treatment modality when a lesion is determined to be mass forming or localized as an abscess due to fast recovery and high success rate | |
| Kayahan et al. (53) | Breast Care | 2012 | 31 | Retrospective cohort study | N/A | Analyzed the treatment methods of surgical excision. Abscess drainage, or steroid therapy | Authors concluded that excision was a superior treatment modality compared to steroid therapy by providing less complications and faster healing | |
| Sheybani et al. (34) | The American College of Obstetricians and Gynecologists | 2015 | 22 | Prospective cohort study | N/A | Compared oral steroids to oral steroids plus methotrexate | Concluded the treatment of choice is corticosteroids and methotrexate, with or without surgery | |
| Pandey et al. (5) | The Breast | 2014 | 49 | Prospective case series | N/A | Compared the treatment modalities of observation, surgery, and steroid therapy | Concluded that the majority of women did not need surgical treatment; recommended observation for painless cases and steroid therapy as an effective nonsurgical option | |
| Karanlik et al. (33) | Breast Care (Basel) | 2014 | 60 | Prospective, non-randomized observational study | N/A | Analyzed low-dose oral corticosteroid therapy alone to low-dose corticosteroid therapy followed by surgery | Concluded that surgical excision after steroid therapy is more advantageous treatment option than steroid therapy alone | |
| Aghajanzadeh et al. (25) | The Breast | 2015 | 206 | Retrospective chart review | N/A | Compared the treatments of surgical excision, oral steroids, steroids plus methotrexate, steroids plus bromocriptine, and surgery plus steroids plus antibiotics | Authors recommended oral corticosteroids as the first line of treatment | |
| Tang et al. (39) | Journal of Surgical Research | 2020 | 49 | Retrospective cohort study | N/A | Compared the treatment modalities of observation, steroid injections, and surgical excision | Authors concluded that intralesional steroid injection is an effective treatment; surgical resection is not necessary for most patients | |
| Yabanoglu et al. (16) | The Breast | 2015 | 77 | Comparative study | N/A | Authors compared conservative management (steroids) vs. surgical excision | Found that wide surgical excision is the preferred approach for treating patients with IGM because of the low recurrence rate | |
| Shin et al. (28) | BMC Women’s Health | 2017 | 34 | Retrospective chart review | N/A | Compared the different treatment modalities of wide excision, steroids after incision and drainage, and antibiotic therapy | Steroid therapy with or without abscess drainage may be the first choice of treatment; recommended against wide excision |
Review results
Diagnosis
Presentation
IGM presents clinically as a palpable breast lesion(s) (Figure 1) that vary in size from 1–5 cm, with an array of other symptoms such as tenderness, overlying skin induration, erythema, sinus tract formation, or breast edema which can clinically mimic a breast abscess or breast cancer on various imaging modalities (1,3,30-32,54-56). IGM commonly presents unilaterally (3) in the upper outer quadrant of the breast (3,4,31), but can be found in any quadrant as well as in both breasts concurrently (27). The palpable, erythematous breast lesion(s) are sterile abscesses often accompanied by the development of spontaneous fistulae or sinus formation.
Inflammation of Cooper’s ligaments can occur, causing nipple inversion or retraction (9). In 13% to 40% of women, enlarged, palpable reactive lymph nodes are noted on exam (3,4,56). Systemic symptoms, such as a fever, are generally absent from the objective findings (8). An essential component of this challenging disease is recognizing the complex presentation to avoid unnecessary surgical interventions and excessive antibiotics. Clinicians diagnose IGM when all other differential diagnoses are excluded, and specific pathologic findings on core needle biopsy are present.
Imaging
In addition to the clinical symptoms mimicking other diseases, the imaging can also present ambiguously. Mammography findings can include an ill-defined density with speculated margins along with associated overlying skin thickening (Figure 2) (56,57). On ultrasound, IGM displays abscess formation with tubular extensions or hypoechoic mass (Figure 3A), with or without enlarged lymph nodes (Figure 3B) with mild concentric cortical thickening (3,56,57). Magnetic Resonance Imaging (MRI) shows IGM as one or many masses with a ring or nodular enhancement (Figure 4) (57). Since IGM is a chronic disease that needs monitoring for a significant amount of time, MRI’s can help evaluate the extent of the disease at presentation as well as monitor progression following the reduction and resolution of lesions with time (9).


Biopsies
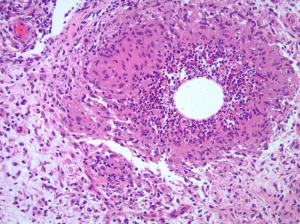
IGM is confirmed with specific histopathological findings obtained from a core needle biopsy of the abscess wall. The hallmark pathological finding of IGM is the presence of multinucleated giant cells, plasma cells, polymorphonuclear leukocytes, lymphocytes, and occasionally sterile microabscesses (Figure 5) (3,4,31,56,58). A core needle biopsy is preferred over an excisional biopsy or fine-needle aspiration (FNA) because it completely characterizes the lesion, is less disfiguring and rules out malignancy (31). If an abscess is present, drainage should occur by FNA and cultures should be obtained to rule out any infectious etiology such as aerobes, anaerobes, fungal infection (periodic acid-Schiff), as well as microorganisms such as Corynebacterium (Gram stain), or acid-fast bacilli (Zeihl-Neelsen) (3,59). Other plausible pathogens should be ruled out at this time including mycobacterial, parasitic, or mycotic origins (1,9,27). Tuberculosis mastitis should also be eliminated from the differential diagnosis through a purified protein derivative (PPD) serum test, skin test, or chest x-ray in addition to the tissue staining for the acid-fast bacilli. Autoimmune etiologies like sarcoidosis or foreign material should be excluded through blood work examining antinuclear antibody (ANA), rheumatoid factor, C-reactive protein, and alpha-I-antitrypsin (1,9,27).
Etiology
The etiology of IGM remains unclear, although a correlation is well documented with recently pregnant or lactating women (51). Many studies support the hypothesis of an autoimmune component (1,9). Other theories suggest an association of hormonal imbalance, oral contraceptives (31), smoking (60), corynebacterium (28), unknown microbiological agents (8), and α1-antitrypsin deficiency (8) whereas other studies have refuted these associations (8,30). It is speculated that the pathology is from local trauma or changes to the ductal epithelium allowing for luminal secretions to be present in the lobular connective tissue, therefore causing lymphocyte and macrophage migration, triggering a granulomatous response within the breast (9). Further research is needed to isolate the exact mechanism of IGM, which would allow for a more personalized treatment approach.
Treatment
Currently, no standardized treatment for IGM exists. Treatments are decided through analyzing each patient’s presentation, the severity of the symptoms, the size of the lesions, the overall health of the patient as well as the surgeon’s preferred treatment method. Many treatment algorithms are present throughout the literature, but none have been widely accepted (3,4,27,31,54,55,61). Although a benign disease, effective treatment is necessary to manage the symptoms and prevent the recurrence of the disease. Recurrence and relapse rates for IGM have varied from 20–50% (3,57,62) causing stress for the patient and the treating physician. Importantly and reassuringly, IGM is a self-limiting disease resolving within 2 to 24 months of onset, regardless of the treatment modality used (4,57). A variety of treatment methods have been utilized to relieve the patient’s chief complaints such as observation, various forms of steroids, immune modulators and surgical excision. This review will outline each treatment method and give an algorithm up-to-date with the current research. An extensive literature review of IGM treatment modalities is presented (Table 1).
Antibiotics and pain management
Since IGM is a disease of exclusion, it is essential upon presentation to rule out any infectious agents while beginning a workup, as bacterial abscesses are more common. Therefore, a bacterial etiology must be before diagnosing IGM. All abscesses should be aspirated or drained followed by broad-spectrum antibiotics during the period of diagnostic evaluation. Acceptable antibiotics include sulfamethoxazole and trimethoprim, amoxicillin/clavulanate (4), doxycycline (48) as well as metronidazole.
For symptomatic pain relief, nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen (Advil) and celecoxib (Celebrex) are the drugs of choice to help with the inflammatory pain that corresponds to the IGM masses (3). Opiates are rarely required for this level of pain management (3).
Observation
IGM is known to be self-limiting; therefore a more conservative treatment method used is observation of the abscesses with regular clinic examinations (14). Many patients present with an array of symptoms and abscess sizes, but if the symptoms are mild to painless along with small lesions, observation may be the first line of treatment, as it will self-resolve (5,11-13,15). Patients need to be reassured that IGM does resolve, but the time to resolution can average 5 months (15). Once the diagnosis of IGM is confirmed, and infections etiology including Corynebacterium is ruled out, patients do not necessarily need to maintain on long-term antibiotics. However, physicians also need to follow patients during observation to ensure no signs of other opportunistic bacterial infection occur due to the open skin areas.
Surgical treatment
Surgical treatment methods vary from abscess drainage, wide surgical excision, and mastectomy. Although the literature has demonstrated that wide surgical excision has been successful (4,14,16,17,20,30,31,53,63), recurrence rates range from 0% to 50% (16,30,64). When completing a wide excision, the goal is a complete removal of the questionable area including fistula tract, affected skin, with a lesion free margin ranging from 5 to 10 mm (3,30).
Much debate still exists about the benefits of surgical excision. Since newer treatment methods have evolved within the last ten years, the utilization of surgical intervention as a first-line therapy has reduced (5,13,28,29). If the patient has a single small lesion, surgical excision could be considered but high recurrence rates are likely. Additionally, there are side effects to surgical excision such as poor cosmetic results, extensive scarring, as well as delays in both would healing and fistula formation (28,32,33). Although mastectomy in severe cases has been described, so has recurrence after mastectomy, so this approach is not optimal.
Steroids
The utilization of steroids for IGM was first published in 1980 (65) and remains today the first-line treatment. Steroids are administered in three different ways: oral, topical, or injections.
Oral steroids are still commonly used and have documented success rates up to 80% (22), but still have the chance for relapse. The prescribed range for IGM is 10–60 mg/day of prednisone or 30–60 mg/day of prednisolone (27) with a gradual taper over the following weeks to months (30). Additional treatment options include 16 mg prednisolone twice a day for two weeks (36) or 1 mg/kg of prednisolone for three weeks (3). Unfortunately, the high rates of IGM resolution with use of oral steroids come at a price: systemic side effects. The side effects range from glucose intolerance and insomnia to cushingoid features. In addition, patients can potentially relapse when discontinuing treatment a few months later (3), however an extra course of steroids can be prescribed when that occurs.
Clinicians prescribe topical steroids as a popular alternative to oral steroids due to having less systemic side effects. Case reports all show positive results and endorse the usage of topical steroids (23,36-38). The pharmacological agent of choice is prednisolone ointment (0.125%) twice a day for 1–3 months (23,36). The recurrence rates for topical steroid use range from 10–18% (23,36-38). In a study published by Cetin et al., the steroid administration routes of topical, systemic, and topical plus systemic steroids were examined and topical steroids had the best results with the lowest recurrence rate of 14.7% (23).
Steroid injections directly into the IGM lesions are the newest advancements in the treatment of IGM and given the reported effectiveness and decreased recurrence rates (39-42), should be considered as the first-line therapy for any newly presenting IGM patient. Steroid injections are based on the well-established techniques for treating arthritic diseases. Steroid injections for IGM were first described in 2012 with a small sample size, but a very low recurrence rate (42). Many newer studies have reported no recurrences with steroid injections along with a faster resolution time compared to other treatment modalities (39-42). The prescribed dose is Kenalog-40 (triamcinolone 40 mg/mL) ranging in volume from 2–4 mL (80–160 mg) mixed with lidocaine as a local anesthetic (39). The injection into the IGM lesion(s) is completed under ultrasound guidance and repeated every 1–2 weeks until resolution. A potential complication of this treatment modality is skin atrophy (39), but otherwise it holds a great deal of promise as a minimally invasive first-line treatment approach.
Immune modulators
Methotrexate (MTX), an immunosuppressive agent, has also been successful in the treatment of IGM. This method is an alternate therapeutic option for any patient who is unresponsive to steroid therapy (3,49). MTX can be given with other treatment modalities such as steroids (25), but a recent study has shown promising effects of this treatment modality on its own demonstrating that by 15 months of treatment, 94% of patients had disease improvement along with 75% achieving remission (50). The pharmacological dosing for MTX is 10–15 mg/week and increasing to 20–25 mg/week given either orally or subcutaneously based on clinical response (50). Reported adverse effects range from elevated liver enzymes and hair loss to more mild symptoms such as nausea, decreased appetite, and mild headaches (41,50).
In the situation where the IGM lesions are not responding (or progressing) and MTX is being considered, consultation to rheumatology and infectious disease is recommended.
Studies comparing different treatment modalities
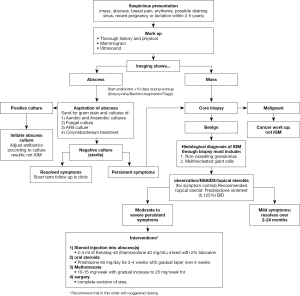
A comprehensive search was conducted over all the published literature for IGM examining the many detailed treatment studies. A comprehensive table (Table 1) is provided of the most significant treatment studies along with all comparative studies to date (full review: https://cdn.amegroups.cn/static/public/ABS-2020-BBD-03-supplementary.xlsx). Based on review of these comparisons, a flow sheet reviewing work-up and management options has been complied as a reference (Figure 6).
Discussion
Many significant advancements in the treatment modalities for IGM have occurred within the last ten years. Still, randomized controlled trials are needed to determine which treatment modality is superior. Promising studies exist for each treatment modality, but an accurate conclusion about the best treatment modality cannot be drawn without a randomized control trial. As highlighted in the spreadsheet of treatment studies (Table 1), the recurrence rates for steroid injections into the abscess cavity are remarkably low in the published literature. Due to these low rates, steroid injections hold great promise and; therefore, we recommend it as the preferred treatment modality due to the high success, minimal systemic side effects and low recurrence rates.
Our comprehensive treatment algorithm (Figure 6) serves to provide a road map for any clinician to diagnose and treat IGM successfully. A thorough workup is critical to exclude all other differential diagnoses to detect IGM accurately. From our literature review, a comprehensive treatment plan is presented to help guide any clinician who encounters IGM in their clinic. Patients presenting with a mass suspicious for IGM should have diagnostic imaging. If an abscess is present, aspiration should be performed, sent for cultures and prophylactic antibiotics initiated. If the abscess cultures are sterile or there is a mass on imaging, a core biopsy should be performed to obtain a tissue diagnosis to confirm IGM. Based on the literature review, the authors recommend observation as the first line treatment with attention to symptom management. This includes non-steroidal anti-inflammatory medication for the pain and topic steroids to the affected area. If the symptoms worsen, use of injectable steroids into the abscess cavity can facility resolution of the breast abscesses. If no improvement or a large area of the breast is involved, oral steroids can be utilized. In refractory cases, methotrexate can be used. Surgical excision should be used sparingly, with mastectomy as a very last resort.
Acknowledgments
Pathology photo Figure 5 courtesy of Dr. J. Jordi Rowe, Department of Pathology, Cleveland Clinic.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Katharine Yao) for the series “A Practical Guide to Management of Benign Breast Disease” published Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form, available at: http://dx.doi.org/10.21037/abs-20-89. The series “A Practical Guide to Management of Benign Breast Disease” was commissioned by the editorial office without any funding or sponsorship. Both authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kessler E, Wolloch Y. Granulomatous Mastitis: A Lesion Clinically Simulating Carcinoma. Am J Clin Pathol 1972;58:642-6. [Crossref] [PubMed]
- Ocal K, Dag A, Turkmenoglu O, et al. Granulomatous Mastitis: Clinical, Pathological Features, and Management. Breast J 2010;16:176-82. [Crossref] [PubMed]
- Joseph KA, Luu X, Mor A. Granulomatous Mastitis: A New York Public Hospital Experience. Ann Surg Oncol 2014;21:4159-63. [Crossref] [PubMed]
- Kiyak G, Dumlu EG, Kilinc I, et al. Management of idiopathic granulomatous mastitis: Dilemmas in diagnosis and treatment. BMC Surg 2014;14:66. [Crossref] [PubMed]
- Pandey TS, Mackinnon JC, Bressler L, et al. Idiopathic Granulomatous Mastitis-A Prospective Study of 49 Women and Treatment Outcomes with Steroid Therapy. Breast J 2014;20:258-66. [Crossref] [PubMed]
- Mohammed S, Statz A, Lacross JS, et al. Granulomatous mastitis: A 10 year experience from a large inner city county hospital. J Surg Res 2013;184:299-303. [Crossref] [PubMed]
- Barreto DS, Sedgwick EL, Nagi CS, et al. Granulomatous mastitis: etiology, imaging, pathology, treatment, and clinical findings. Breast Cancer Research and Treatment 2018;171:527-34. [Crossref] [PubMed]
- Altintoprak F, Kivilcim T, Ozkan OV. Aetiology of idiopathic granulomatous mastitis. World J Clin Cases 2014;2:852-8. [Crossref] [PubMed]
- Schelfout K, Tjalma WA, Cooremans ID, et al. Observations of an idiopathic granulomatous mastitis. Eur J Obstet Gynecol Reprod Biol 2001;97:260-2. [Crossref] [PubMed]
- Tang A, Dominguez DA, Edquilang JK, et al. Association for Academic Surgery Granulomatous Mastitis: Comparison of Novel Treatment of Steroid Injection and Current Management. J Surg Res 2020;300-5. [Crossref] [PubMed]
- Ma X, Min X, Yao C. Different Treatments for Granulomatous Lobular Mastitis: A Systematic Review and Meta-Analysis. Breast Care 2020;15:60-6. [Crossref] [PubMed]
- Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg 2015;210:258-62. [Crossref] [PubMed]
- Mahlab-Guri K, Asher I, Allweis T, et al. Granulomatous Lobular Mastitis. Isr Med Assoc J 2015;17:476-80. [PubMed]
- Hur SM, Cho DH, Lee SK, et al. Experience of treatment of patients with granulomatous lobular mastitis. J Korean Surg Soc 2013;85:1-6. [Crossref] [PubMed]
- Davis J, Cocco D, Matz S, et al. Re-evaluating if observation continues to be the best management of idiopathic granulomatous mastitis. Surgery 2019;166:1176-80. [Crossref] [PubMed]
- Yabanoğlu H, Colakoglu TC, Belli S, et al. A Comparative Study of Conservative versus Surgical Treatment Protocols for 77 Patients with Idiopathic Granulomatous Mastitis. Breast J 2015;21:363-9. [Crossref] [PubMed]
- Alrayes A, Almarzooq R, Abdulla HA. Surgical treatment of granulomatous mastitis: Our experience in Bahrain. Breast J 2019;25:958-62. [Crossref] [PubMed]
- Korkut E, Akcay MN, Karadeniz E, et al. Granülomatöz mastit: Bir üniversite hastanesinde on yıllık deneyim. Eurasian J Med 2015;47:165-73. [Crossref] [PubMed]
- Erozgen F, Ersoy YE, Akaydin M, et al. Corticosteroid treatment and timing of surgery in idiopathic granulomatous mastitis confusing with breast carcinoma. Breast Cancer Res Treat 2010;123:447-52. [Crossref] [PubMed]
- Kok KYY, Telisinghe PU. Granulomatous mastitis: Presentation, treatment and outcome in 43 patients. Surgeon 2010;8:197-201. [Crossref] [PubMed]
- Yau FM, MacAdam SA, Kuusk U, et al. The surgical management of granulomatous mastitis. Ann Plast Surg 2010;64:9-16. [Crossref] [PubMed]
- Deng JQ, Yu L, Yang Y, et al. Steroids administered after vacuum-assisted biopsy in the management of idiopathic granulomatous mastitis. J Clin Pathol 2017;70:827-31. [Crossref] [PubMed]
- Çetin K, Sıkar HE, Göret NE, et al. Comparison of Topical, Systemic, and Combined Therapy with Steroids on Idiopathic Granulomatous Mastitis: A Prospective Randomized Study. World J Surg 2019;43:2865-73. [Crossref] [PubMed]
- Mahmodlou R, Dadkhah N, Abbasi F, et al. Idiopathic granulomatous mastitis: dilemmas in diagnosis and treatment. Electron Physician 2017;9:5375-9. [Crossref] [PubMed]
- Aghajanzadeh M, Hassanzadeh R, Alizadeh Sefat S, et al. Granulomatous mastitis: Presentations, diagnosis, treatment and outcome in 206 patients from the north of Iran. Breast 2015;24:456-60. [Crossref] [PubMed]
- Montazer Majid. Maryam Dadashzadeh SEMT. Comparison of the Outcome of Low Dose and High-Dose Corticosteroid in the Treatment of Idiopathic Granulomatous Mastitis. Asian Pac J Cancer Prev 2020;21:993-6. [Crossref] [PubMed]
- Mizrakli T, Velidedeoglu M, Yemisen M, et al. Corticosteroid treatment in the management of idiopathic granulomatous mastitis to avoid unnecessary surgery. Surg Today 2015;45:457-65. [Crossref] [PubMed]
- Shin YD, Park SS, Song YJ, et al. Is surgical excision necessary for the treatment of Granulomatous lobular mastitis? BMC Womens Health 2017;17:49. [Crossref] [PubMed]
- Wang J, Zhang Y, Lu X, et al. Idiopathic Granulomatous Mastitis with Skin Rupture: A Retrospective Cohort Study of 200 Patients Who Underwent Surgical and Nonsurgical Treatment. J Investig Surg 2019:1-6.
- Akcan A, Oz AB, Dogan S, et al. Idiopathic Granulomatous Mastitis: Comparison of Wide Local Excision with or without Corticosteroid Therapy. Breast Care (Basel) 2014;9:111-5. [Crossref] [PubMed]
- Oran EŞ, Gürdal SÖ, Yankol Y, et al. Management of Idiopathic Granulomatous Mastitis Diagnosed by Core Biopsy: A Retrospective Multicenter Study. Breast J 2013;19:411-8. [Crossref] [PubMed]
- Gurleyik G, Aktekin A, Aker F, et al. Medical and surgical treatment of idiopathic granulomatous lobular mastitis: A benign inflammatory disease mimicking invasive carcinoma. J Breast Cancer 2012;15:119-23. [Crossref] [PubMed]
- Karanlik H, Ozgur I, Simsek S, et al. Can steroids plus surgery become a first-line treatment of idiopathic granulomatous mastitis? Breast Care 2014;9:338-42. [Crossref] [PubMed]
- Sheybani F, Sarvghad M, Naderi H, et al. Treatment for and Clinical Characteristics of Granulomatous Mastitis. Obstet Gynecol 2015;125:801-7. [Crossref] [PubMed]
- Kim J, Tymms K, Buckingham J. Methotrexate in the Management of Granulomatous Mastitis. Anz J Surg 2003;73:247-9. [Crossref] [PubMed]
- Altintoprak F, Kivilcim T, Yalkin O, et al. Topical Steroids Are Effective in the Treatment of Idiopathic Granulomatous Mastitis. World J Surg 2015;39:2718-23. [Crossref] [PubMed]
- Altintoprak F. Topical steroids to treat granulomatous mastitis: A case report. Korean J Intern Med 2011;26:356-9. [Crossref] [PubMed]
- Gunduz Y, Altintoprak F, Tatli Ayhan L, et al. Effect of Topical Steroid Treatment on Idiopathic Granulomatous Mastitis: Clinical and Radiologic Evaluation. Breast J 2014;20:586-91. [Crossref] [PubMed]
- Tang A, Dominguez DA, Edquilang JK, et al. Granulomatous Mastitis: Comparison of Novel Treatment of Steroid Injection and Current Management. J Surg Res 2020;254:300-5. [Crossref] [PubMed]
- Kim BS, Bon Y. Usefulness of Ultrasound-guided Intralesional Steroid Injection in Management of Idiopathic Granulomatous Mastitis. J Surg Ultrasound 2016;3:40-5.
- Alper F, Karadeniz E, Güven F, et al. The evaluation of the efficacy of local steroid administration in idiopathic granulomatous mastitis: The preliminary results. Breast J 2020;26:309-11. [Crossref] [PubMed]
- Munot K, Nicholson S, Birkett V. Granulomatous mastitis - A novel method of treatment. Eur J Surg Oncol 2012;38:461-2. [Crossref]
- Raj N, Macmillan RD, Ellis IO, et al. Rheumatologists and breasts: immunosuppressive therapy for granulomatous mastitis. Rheumatology (Oxford) 2004;43:1055-6. [Crossref] [PubMed]
- Wang ST, Lin JC, Li CF, et al. A successful case of etanercept used for idiopathic granulomatous mastitis. Breast J 2019;25:343-5. [Crossref] [PubMed]
- Schmajuk G, Genovese MC. First report of idiopathic granulomatous mastitis treated with methotrexate monotherapy. J Rheumatol 2009;36:1559. [Crossref] [PubMed]
- Farouk O, Abdelkhalek M, Abdallah A, et al. Rifampicin for Idiopathic Granulomatous Lobular Mastitis: A Promising Alternative for Treatment. World J Surg 2017;41:1313-21. [Crossref] [PubMed]
- Akbulut S, Arikanoglu Z, Senol A, et al. Is methotrexate an acceptable treatment in the management of idiopathic granulomatous mastitis? Arch Gynecol Obstet 2011;284:1189-95. [Crossref] [PubMed]
- Steuer AB, Stern MJ, Cobos G, et al. Clinical Characteristics and Medical Management of Idiopathic Granulomatous Mastitis. JAMA Dermatol 2020;156:460-4. [Crossref] [PubMed]
- Haddad M, Sheybani F, Arian M, et al. Methotrexate‐based regimen as initial treatment of patients with idiopathic granulomatous mastitis. Breast J 2020;26:325-7. [Crossref] [PubMed]
- Postolova A, Troxell ML, Wapnir IL, et al. Methotrexate in the treatment of idiopathic granulomatous mastitis. J Rheumatol 2020;47:924-7. [Crossref] [PubMed]
- Azizi Armina. Idiopathic granulomatous mastitis: Management and predictors of recurrence in 474 patients. Breast J 2020;26:1358-62. [Crossref] [PubMed]
- Atak T, Sagiroglu J, Eren T, et al. Strategies to treat idiopathic granulomatous mastitis: Retrospective analysis of 40 patients. Breast Dis 2015;35:19-24. [Crossref] [PubMed]
- Kayahan M, Kadioglu H, Muslumanoglu M. Breast Care Management of Patients with Granulomatous Mastitis: Analysis of 31 Cases. Breast Care 2012;7:226-30. [Crossref] [PubMed]
- Baslaim MM, Hind AE, Khayat A, et al. Idiopathic Granulomatous Mastitis: A Heterogeneous Disease with Variable Clinical Presentation. World J Surg 2007;31:1677-81. [Crossref] [PubMed]
- Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: In search of a therapeutic paradigm. Am Surg 2007;73:798-802. [Crossref] [PubMed]
- Boufettal H, Essodegui F, Noun M, et al. Idiopathic granulomatous mastitis: A report of twenty cases. Diagn Interv Imaging 2012;93:586-96. [Crossref] [PubMed]
- Gautier N, Lalonde L, Tran-Thanh D, et al. Chronic granulomatous mastitis: Imaging, pathology and management. Eur J Radiol 2013;82:e165-75. [Crossref] [PubMed]
- Fletcher A, Magrath IM, Riddell RH, et al. Granulomatous mastitis: a report of seven cases. J Clin Pathol 1982;35:941-5. [Crossref] [PubMed]
- Stary CM, Lee YS, Balfour J. Idiopathic granulomatous mastitis associated with corynebacterium sp. Infection. Hawaii Med J 2011;70:99-101. [PubMed]
- Koksal H, Vatansev H, Artac H, et al. The clinical value of interleukins-8, -10, and -17 in idiopathic granulomatous mastitis. Clin Rheumatol 2020;39:1671-7. [Crossref] [PubMed]
- Seo HRN, Na KY, Yim HE, et al. Differential diagnosis in idiopathic granulomatous mastitis and tuberculous mastitis. J Breast Cancer 2012;15:111-8. [Crossref] [PubMed]
- Lai EC, Chan WC, Ma TK, et al. The Role of Conservative Treatment in Idiopathic Granulomatous Mastitis. Breast J 2005;11:454-6. [Crossref] [PubMed]
- Taghizadeh R, Shelley OP, Chew BK, et al. Idiopathic Granulomatous Mastitis: Surgery, Treatment, and Reconstruction. Breast J 2007;13:509-13. [Crossref] [PubMed]
- Patel RA, Strickland P, Sankara IR, et al. Idiopathic granulomatous mastitis: Case reports and review of literature. J Gen Intern Med 2010;25:270-3. [Crossref] [PubMed]
- DeHertogh DA, Rossof AH, Harris AA, et al. Prednisone Management of Granulomatous Mastitis. N Engl J Med 1980;303:799-800. [Crossref] [PubMed]
Cite this article as: Bede K, Valente SA. Idiopathic granulomatous mastitis. Ann Breast Surg 2020;4:24.