
Clinical management of radial scar without atypia diagnosed on core needle biopsy
Introduction
Radial scars (RS), also known as complex sclerosing lesions, are benign proliferative breast lesions and are usually asymptomatic in healthy women. In the pre-mammographic screening era, they usually presented as incidental findings in breast specimens that were surgically excised for unrelated conditions. Since the introduction of screening mammography, RS has increasingly become a screen-detected lesion, and its detection has increased even more with the adoption of digital breast tomosynthesis (DBT). Because of its spiculated appearance on mammography, which can mimic breast cancer, RS is often evaluated with core needle biopsy (CNB).
Because of the significant upgrade risk to malignancy, it is generally accepted that RS with atypia should be excised. The management of RS without atypia is more controversial. The risk of association with malignancy in pure RS ranges widely in the literature and may be related to various patient, imaging, biopsy, and pathologic factors. This paper serves as a review of the clinical evidence regarding the management of CNB-diagnosed pure RS. In addition, we have proposed a management algorithm of these lesions.
Incidence
In autopsy studies, the frequency of RS ranges from 14% to 43%, depending on the amount of tissue examined, and RS is frequently multicentric or bilateral (1,2). The reported incidence of RS from screening mammograms is 0.3–0.9 per 1,000 women screened (3). In a series of 10,921 image-guided needle biopsies, pure RS accounted for 0.8% (4), and in another series of 4,458 consecutive image-guided needle biopsies, it accounted for 1.8% (5).
Imaging
The mammographic diagnosis of RS has been defined by a set of criteria put forth by Tabar and Dean (6): (I) varying appearance in different projections; (II) absence of solid dense central mass; (III) presence of long thin spicules; (IV) radiolucent linear structures parallel to radiopaque spicules (i.e., “black star” appearance); (V) absence of palpable lesions or skin changes. None of these findings is specific for RS, as many studies have shown inaccurate radiologic classification based on some of these criteria (7-9). Since the appearance of RS mimics that of invasive breast cancer despite various imaging criteria, CNB is often required to distinguish the two entities.
Microcalcifications may occur in RS, even though their absence had been suggested in the past to be a feature of RS (10). They are usually associated with benign proliferative changes and are nonspecific with respect to differentiating RS from malignancy. In some cases, the microcalcifications may be the only abnormality on imaging, and RS becomes an incidental pathologic finding upon stereotactic CNB (10-12).
DBT has been shown to detect malignant neoplasms at a higher rate than digital two-dimensional mammography (DM), due to its ability to better characterize masses and architectural distortions (Figure 1). Not surprisingly, the rate of identifying RS on DBT has increased compared to DM (13,14). In Phantana-Angkool et al.’s study of 301,133 consecutive mammograms between 2007 to 2017 (179,085 DM, 122,048 DBT), the detection rate of pure RS significantly increased from 0.04% to 0.13% with DBT (P<0.0001). However, the upgrade rate to malignancy was not statistically different between DM and DBT (6% vs. 3%, P=0.24); nor was the upgrade rate to a high risk lesion (HRL) (12% vs. 22%, P=0.10) (13). The similar rate of upstaging despite an increase in detection with DBT suggests that there are no reliable DBT-specific features to distinguish RS from malignancy even on modern mammography.
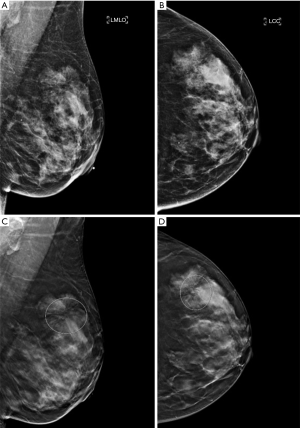
RS may be visible on ultrasound in only half of the cases, and, when visible, often presents as an irregular hypoechoic mass with indistinct margins with or without shadowing (15,16). There are no sonographic features that can reliably differentiate RS from malignancy, but an ultrasound should always be performed when there is an area of architectural distortion on mammography. If a mass is identified on ultrasound, it can serve as the target for ultrasound-guided CNB.
The presentation of RS on breast MRI includes masses (50%), architectural distortions, non-mass lesions, and single foci, with variable enhancement patterns (17). Just as in mammography and ultrasonography, most MRI characteristics of RS are suspicious. It has been suggested that the absence of MRI enhancement of a lesion suspected to be RS may predict a benign excision result (18-20). In a study of 169 HRLs including 42 RS, the negative predictive value of MRI for RS was 97.6%, and the one false-negative case was upgraded to low grade ductal carcinoma in situ (DCIS) (18). A similarly high negative predictive value of 97% was reported by Londero et al., and only one of the 31 non-enhancing RS was upgraded to low grade DCIS as well (20). Thus, nonsurgical management may be considered for CNB-diagnosed pure RS, when MRI is negative for any abnormal findings. However, the high cost of breast MRI and lack of wide availability limit its usefulness.
Pathology
Histologically, RS is characterized by a fibroelastic core with surrounding radiating spokes of ducts and lobules, which often contain a variety of proliferative changes, such as epithelial hyperplasia, duct ectasia, adenosis, and cysts (3) (Figure 2). These lesions may be associated with epithelial atypia or in situ or invasive carcinoma, and the entrapped central glandular elements, especially when proliferative changes are present, may give the appearance of tubular carcinoma. The benignity of the lesion can be demonstrated by an intact myoepithelial cell layer on immunohistochemistry staining. It is generally accepted that a RS larger than one centimeter on pathology is called a complex sclerosing lesion, whereas lesions 1 cm or smaller are called RS. Although most RS are microscopic findings, some can achieve a size that makes them visible on imaging.

Biologic relevance
Over the years, the biologic relevance of RS and their relationship to breast cancer have been much debated. On the basis of existing literature, there are several “truths” to the nature of RS. First, RS is not a premalignant lesion. The morphologic similarity to carcinoma, especially tubular carcinoma, and the presence of carcinoma in some RS had led to the hypothesis that RS could represent a direct precursor to carcinoma (21,22). That theory has been debunked by several later studies, none of which found transitional features of RS to carcinoma (1,2,23,24).
Second, RS frequently coexists with other proliferative lesions and sometimes carcinoma. The frequency of association with malignancy has been reported to be as high as 45% (25), although the upgrade risk reported in a recent meta-analysis is much lower at 5% or less for pure RS (26). The high malignancy rates have led many to conclude that all RS should be excised, a practice pattern that has been questioned in recent years.
Third, RS does not impart an increased breast cancer risk above that of the associated proliferative changes. A case-control study of 99 women with RS within the Nurses’ Health Study had suggested that RS was an independent risk factor for breast cancer (27). In this study by Jacobs et al., the relative risk (RR) for women with proliferative disease and RS was 3.0, and, for women with proliferative disease alone, was 1.5. The risk was similarly increased for atypia and RS, compared to atypia alone (RR 5.8 vs. 3.8). A much larger study by Sanders et al. as part of the Nashville Breast Cohort showed contrary results (28). In this study of 880 women with RS, the overall risk of breast cancer associated with RS was 1.82, and RS was not found to have an additive risk and was not an independent risk factor for carcinoma. Specifically, the RR associated with proliferative disease alone was 1.74 and was 2.13 when RS was present; RR for atypia with RS was 5.39, not significantly different from RR of 4.38 for atypia alone. Similar findings were seen in a Mayo Clinic study of 439 women with RS, which showed no increased breast cancer risk associated with RS aside from that imparted by the accompanying proliferative changes (29).
Management
The upgrade rate to malignancy at surgical excision of RS diagnosed from CNB varies from 0 to 45% (26) and has led to widely varying practice patterns. Such wide variation in upgrade rates is confounded by the presence of atypia, sampling method, biopsy device and gauge, number of samples, criteria for subsequent excision, and imaging-pathologic concordance. The presence of atypia with RS on CNB significantly increases the malignant upgrade rate (26,30). In a recent meta-analysis of 3,163 RS excised after CNB, the malignant upgrade rate for RS with atypia was 29% but only 5% for pure RS (26). Hence, RS with atypia diagnosed on CNB should be considered for excision. In the United States, surgical excision of RS with high risk features is standard practice. However, management of pure RS remains controversial. In 2016, the American Society of Breast Surgeons released a consensus guideline recommending excision of most RS, although small, well-sampled lesions with large-gauge devices may be followed with imaging (31). However, the lack of consensus on what constitutes a “small” or “well-sampled” lesion in this statement highlights the difficulty in identifying a subset of RS at low enough risk of upgrade as to avoid surgical biopsy.
Table 1 summarizes the upgrade rates to malignancy and HRL including atypical ductal hyperplasia and lobular neoplasia in studies of at least 40 pure CNB-diagnosed RS done in the last 10 years. Whereas some earlier series had shown much higher malignant upgrade rates of up to 25% even for pure RS (47), the rates of malignant upgrade in this table range from 0% to 10.5%, with more recent studies showing lower upgrade rates than earlier ones. In the series from 2016 on, the malignant upgrade rates for pure RS were all 5% or less.
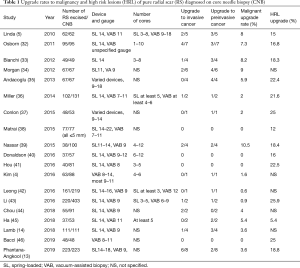
Full table
Stringent exclusion of atypia on CNB and increased extent of sampling likely account for the low upgrade rates in the recent times. Several single-institution series showed larger gauge biopsy devices and more core samples reduced the upgrade rate (47-49). Farshid et al.’s meta-analysis stratified 3,163 RS into three subgroups by CNB gauge, 14 G, a mix of 8–16 G, and vacuum-assisted biopsy (VAB) 8–11 G, and showed a stepwise decrease in malignant upgrade rates for pure RS, 5%, 2%, and 1% respectively (26). It is intuitive that using a larger gauge needle or obtaining more cores would produce more comprehensive sampling of the lesion and hence reduce the upgrade rate upon excision.
Many recent studies, especially those utilizing VAB, have advocated non-surgical management of pure RS. When RS is diagnosed via a spring-loaded device, a repeat biopsy with VAB can be performed, and surgery can be avoided if the repeat biopsy is benign (50,51). In the United Kingdom, it is standard practice that RS with or without atypia diagnosed on CNB undergo vacuum-assisted excision (VAE) for small lesions or thorough sampling with VAB for larger lesions (52). If VAE is benign without atypia, the lesion is observed; if VAE shows atypia, either observation or diagnostic surgery is considered, depending on the extent and degree of atypia. VAB can be a particularly useful tool for large RS, when surgical excision would result in significant deformity.
Other factors that have been associated with upgrade include age older than 53–64 years (34,38,43,45), lesion size larger than 1–2 cm (39,45,53), mammographic appearance with mass or architectural distortion versus calcifications (36), and non-incidental nature of RS (38,46). However, these findings are not consistent in the literature. By far, the strongest and the most consistent risk factor for upgrade is the presence of atypia (26,30).
There is no consensus on an acceptable level of upgrade risk for avoidance of surgery. The BIRADs 3 category as used by the American College of Radiology designates a probably benign mammographic finding that has a 2% or less risk of malignancy (54), and short-term imaging surveillance is usually accepted for this category of lesions. In a meta-analysis of 11,423 high-risk (B3) lesions, the malignant upgrade rate of the 334 pure RS was 6% (30). The authors proposed that a 10% malignant risk was low enough to leave the lesion in the breast, and hence, pure RS could be observed. By these standards, most pure RS diagnosed on CNB in the modern era have a low enough risk of malignant upgrade that observation may be considered.
When breast cancers are detected on surgical excision for RS, they are usually small in size, low grade, hormone receptor-positive, and more likely to be preinvasive than invasive (5,13,36,43,44). If left in situ, these tumors with extremely favorable prognostic features may become apparent on close imaging surveillance, and later detection is unlikely to affect the overall outcome. Additionally, multiple studies showed that unexcised RS that were closely followed did not result in any malignancy, even at 11 years of follow-up (41,45,55), although RS recommended for observation may exhibit different features from RS recommended for surgical excision. The overall low rate of upgrade to malignancy and the extremely favorable features of these tumors suggest that many surgical excisions of RS can be avoided, if the goal of the procedure is only to rule out underlying malignancy.
However, finding associated HRL on surgical excision may have treatment implications. The rates of upgrade to HRL on excision of RS are higher than the malignant upgrade rates, ranging from 12% to 26% in Table 1. In a study of 18 microscopic RS seen only on pathology from CNB and included in their entirety in the biopsy specimens, the malignant upgrade rate was 0, but the HRL rate was 39% (56). Even RS well sampled with VAB have an associated HRL rate of 25% (46). A diagnosis of atypical ductal hyperplasia, atypical lobular hyperplasia, or lobular carcinoma in situ is associated with an increased risk of breast cancer development in either breast. For women at increased risk of breast cancer, risk-reducing strategies may be considered, including chemoprevention agents and potentially risk-reducing surgery (57). Annual breast MRI screening may be considered in addition to annual mammography for women whose lifetime risk of breast cancer is 20% or greater (58). Hence, for women whose management decisions may change as a result of any HRL co-existing with RS, further diagnostic workup of RS with either surgical excision or VAB excision should be considered to rule out any underlying atypia.
There is no arbitrary threshold for surgical excision of pure RS. Each case should be evaluated on an individual basis and ideally be discussed at multidisciplinary tumor board. Assessing imaging-pathology concordance is essential, as is ruling out atypia on CNB. The goal and the rationale for excision should be discussed with every patient with RS, and the decision to excise, observe, or to repeat a biopsy with VAB be made accordingly.
In summary, we propose the following management of pure RS (Figure 3). For (relatively) healthy patients who would benefit from chemoprevention and/or additional imaging screening if concomitant HRL is found, RS of all sizes should be excised; alternatively, VAB excision can be performed. For patients with significant comorbidities or those who would not consider risk reduction strategies, RS 1 cm or smaller or RS of any size sampled with VAB can be considered for observation. Larger RS should undergo surgical excision; as an alternative, repeat thorough sampling or excision with VAB can be considered, and the lesion can be observed if the repeat biopsy does not show carcinoma. If there is radiologic-pathologic discordance, either repeat biopsy or surgical excision should be performed. When carcinoma (invasive or preinvasive) is found on VAB excision, additional oncologic surgery must be performed. RS considered for observation or incompletely excised should undergo imaging follow-up, the interval of which should be discussed with the radiologist.
Conclusions
Pure RS diagnosed on CNB has a low risk of malignant upgrade (5% or less) on surgical excision, but the rate of upgrade to HRL is much higher (12–26%). The upgrade risk significantly increases with the presence of atypia, but can be reduced by sampling the lesion with a larger gauge biopsy needle, more cores, and VAB. RS of all sizes should be considered for surgical or VAB excision, if clinical management would change as a result of associated HRL. Otherwise, small (1 cm or less) or well sampled RS can be observed. Larger RS should be excised but can be considered for observation if repeat biopsy with VAB shows benign findings.
Acknowledgments
The authors thank Dr. Deepa Sheth (University of Chicago) for providing the radiology images, and Drs. Husain Sattar and Lisa Han (University of Chicago) for providing the pathology images.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Katharine Yao) for the series “A Practical Guide to Management of Benign Breast Disease” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-85). The series “A Practical Guide to Management of Benign Breast Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wellings SR, Alpers CE. Subgross pathologic features and incidence of radial scars in the breast. Hum Pathol 1984;15:475-9. [Crossref] [PubMed]
- Nielsen M, Jensen J, Andersen JA. An autopsy study of radial scar in the female breast. Histopathology 1985;9:287-95. [Crossref] [PubMed]
- Kennedy M, Masterson AV, Kerin M, et al. Pathology and clinical relevance of radial scars: a review. J Clin Pathol 2003;56:721-4. [Crossref] [PubMed]
- Kim EM, Hankins A, Cassity J, et al. Isolated radial scar diagnosis by core-needle biopsy: is surgical excision necessary? Springerplus 2016;5:398. [Crossref] [PubMed]
- Linda A, Zuiani C, Furlan A, et al. Radial scars without atypia diagnosed at imaging-guided needle biopsy: how often is associated malignancy found at subsequent surgical excision, and do mammography and sonography predict which lesions are malignant. AJR Am J Roentgenol 2010;194:1146-51. [Crossref] [PubMed]
- Tabar L, Dean PB. Teaching Atlas of Mammography, 3rd Ed. Stuttgart, Germany, Thieme 2001:93-147.
- Frouge C, Tristant H, Guinebretiere J, et al. Mammograhpic lesions suggestive of radial scars: microscopic findings in 40 cases. Radiology 1995;195:623-5. [Crossref] [PubMed]
- Mitnick JS, Vazquez MF, Harris MN, et al. Differentiation of radial scar from scirrhous carcinoma of the breast: mammographic-pathologic correlation. Radiology 1989;173:697-700. [Crossref] [PubMed]
- Alleva DQ, Smetherman DH, Farr GH Jr, et al. Radial scar: radiologic-pathologic correlation in 22 case. RadioGraphics 1999;19:S27-S35. [Crossref] [PubMed]
- Orel SG, Evers K, Yeh IT, et al. Radial scar with microcalcifications: radiologic-pathologic correlation. Radiology 1992;183:479-82. [Crossref] [PubMed]
- Patel A, Steel Y, McKenzie J, et al. Raidal scars: a review of 30 cases. Eur J Surg Oncol 1997;23:202-5. [Crossref] [PubMed]
- Burnett SJ, Ng YY, Perry NM, et al. Benign biopsies in the prevalent round of breast screening: a review of 137 cases. Clin Radiol 1995;50:254-8. [Crossref] [PubMed]
- Phantana-Angkool A, Forster MR, Warren YE, et al. Rate of radial scars by core biopsy and upgrading to malignancy or high-risk lesions before and after introduction of digital breast tomosynthesis. Breast Cancer Res Treat 2019;173:23-9. [Crossref] [PubMed]
- Lamb LR, Bhal M, Hughes KS, et al. Pathologic upgrade rates of high-risk breast lesions on digital two-dimensional vs tomosynthesis mammography. J Am Coll Surg 2018;226:858-67. [Crossref] [PubMed]
- Cawson JN. Can sonography be used to help differentiate between radial scars and breast cancers? Breast 2005;14:352-9. [Crossref] [PubMed]
- Cohen MA, Sferlazza SJ. Role of sonography in evaluation of radial scars of the breast. AJR Am J Roentgenol 2000;174:1075-8. [Crossref] [PubMed]
- Linda A, Zuiani C, Londero V, et al. Magnetic resonance imaging of radial sclerosing lesions (radial scars) of the breast. Eur J Radiol 2012;81:3201-7. [Crossref] [PubMed]
- Linda A, Zuiani C, Furlan A, et al. Non-surgical management of high-risk lesions diagnosed at core needle biopsy: can malignancy be ruled out safely with breast MRI? AJR Am J Roentgenol 2012;198:272-80. [Crossref] [PubMed]
- Pediconi F, Occhiato R, Venditti F, et al. Radial scars of the breast: contrast-enhanced magnetic resonance mammography appearance. Breast J 2005;11:23-8. [Crossref] [PubMed]
- Londero V, Zuiani C, Linda A, et al. High-risk breast lesions at imaging-guided needle biopsy: usefulness of MRI for treatment decision. AJR Am J Roentgenol 2012;199:W240-50 [Crossref] [PubMed]
- Fisher ER, Palekar AS, Kotwal N, et al. A nonenapsulated sclerosing lesion of the breast. Am J Clin Pathol 1979;71:240-6. [Crossref] [PubMed]
- Linell F, Ljungberg O, Andersson I. Breast Carcinoma. Aspects of early stages, progression and related problems. Acta Pathol Microbiol Scand Suppl 1980;272:1-233. [PubMed]
- Anderson TJ, Battersby S. Radial scars of benign and malignant breasts: comparative features and significance. J Pathol 1985;147:23-32. [Crossref] [PubMed]
- Nielsen M, Christensen L, Andersen J. Radial scars in women with breast cancer. Cancer 1987;59:1019-25. [Crossref] [PubMed]
- Kirwan SE, Denton ER, Nash RM, et al. Multiple 14G stereotactic core biopsies in the diagnosis of mammographically detected stellate lesions of the breast. Clin Radiol 2000;55:763-6. [Crossref] [PubMed]
- Farshid G, Buckley E. Meta-analysis of upgrade rates in 3163 radial scars excised after needle core biopsy diagnosis. Breast Cancer Res Treat 2019;174:165-77. [Crossref] [PubMed]
- Jacobs TW, Byrne C, Colditz G, et al. Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N Engl J Med 1999;340:430-6. [Crossref] [PubMed]
- Sanders ME, Page DL, Simpson JF, et al. Interdependence of radial scar and proliferative disease with respect to invasive breast carcinoma risk in patients with benign breast biopsies. Cancer 2006;106:1453-61. [Crossref] [PubMed]
- Berg JC, Visscher DW, Vierkant RA, et al. Breast cancer risk in women with radial scars in benign breast biopsies. Breast Cancer Res Treat 2008;108:167-74. [Crossref] [PubMed]
- Forester ND, Lowes S, Mitchell E, et al. High risk (B3) breast lesions: What is the incidence of malignancy for individual lesion subtypes? A systematic review and meta-analysis. Eur J Surg Oncol 2019;45:519-27. [Crossref] [PubMed]
- Consensus guideline on concordance assessment of image-guided breast biopsies and management of borderline or high-risk lesions. American Society of Breast Surgeons [published 2 November 2016, accessed 10 July 2020]. Available online: https://www.breastsurgeons.org/docs/statements/Consensus-Guideline-on-Concordance-Assessment-of-Image-Guided-Breast-Biopsies.pdf
- Osborn G, Wilton F, Stevens G, et al. A review of needle core biopsy diagnosed radial scars in the Welsh Breast Screening Programme. Ann R Coll Surg Engl 2011;93:123-6. [Crossref] [PubMed]
- Bianchi S, Giannotti E, Vanzi E, et al. Radial scar without associated atypical epithelial proliferation on image-guided 14-gauge needle core biopsy: analysis of 49 cases from a single-centre and review of the literature. Breast 2012;21:159-64. [Crossref] [PubMed]
- Morgan C, Shah ZA, Hamilton R, et al. The radial scar of the breast diagnosed at core needle biopsy. Proc (Bayl Univ Med Cent) 2012;25:3-5. [Crossref] [PubMed]
- Andacoglu O, Kanbour-Shakir A, Teh YC, et al. Rationale of excisional biopsy after the diagnosis of benign radial scar on core biopsy: a single institutional outcome analysis. Am J Clin Oncol 2013;36:7-11. [Crossref] [PubMed]
- Miller CL, West JA, Bettini AC, et al. Surgical excision of radial scars diagnosed by core biopsy may help predict future risk of breast cancer. Breast Cancer Res Treat 2014;145:331-8. [Crossref] [PubMed]
- Conlon N, D’Arcy C, Kaplan JB, et al. Radial scar at image-guided needle biopsy. Am J Surg Pathol 2015;39:779-85. [Crossref] [PubMed]
- Matrai C, D’Alfonso TM, Pharmer L, et al. Advocating nonsurgical management of patients with small, incidental radial scars at the time of needle core biopsy: a study of 77 cases. Arch Pathol Lab Med 2015;139:1137-42. [Crossref] [PubMed]
- Nassar A, Conners AL, Celik B, et al. Radial scar/complex sclerosing lesions: a clinicopathologic correlation study from a single institution. Ann Diagn Pathol 2015;19:24-8. [Crossref] [PubMed]
- Donaldson AR, Sieck L, Booth CN, et al. Radial scars diagnosed on breast core biopsy: frequency of atypia and carcinoma on excision and implications for management. Breast 2016;30:201-7. [Crossref] [PubMed]
- Hou Y, Hooda S, Li Z. Surgical excision outcome after radial scar without atypical proliferative lesion on breast core needle biopsy: a single institutional analysis. Ann Diagn Pathol 2016;21:35-8. [Crossref] [PubMed]
- Leong RY, Kohli MK, Zeizafoun N, et al. Radial scar at percutaneous breast biopsy that does not require surgery. J Am Coll Surg 2016;223:712-6. [Crossref] [PubMed]
- Li Z, Ranade A, Zhao C, et al. Pathologic findings of follow-up surgical excision for radial scar on breast core needle biopsy. Hum Pathol 2016;48:76-80. [Crossref] [PubMed]
- Chou WY, Veis DJ, Aft R. Radial scar on image-guided breast biopsy: is surgical excision necessary? Breast Cancer Res Treat 2018;170:313-20. [Crossref] [PubMed]
- Ha SM, Cha JH, Shin HJ, et al. Radial scars/complex sclerosing lesions of the breast: radiologic and clinicopathologic correlation BMC Med Imaging 2018;18:39. [Crossref] [PubMed]
- Bacci J, MacGrogan G, Alran L, et al. Management of radial scars/complex sclerosing lesions of the breast diagnosed on vacuum-assisted large-core biopsy: is surgery always necessary? Histopathology 2019;75:900-15. [Crossref] [PubMed]
- Becker L, Trop I, David J, et al. Management of radial scars found at percutaneous breast biopsy. Can Assoc Radiol J 2006;57:72-8. [PubMed]
- Ferreira AI, Borges S, Sousa A, et al. Radial scar of the breast: Is it possible to avoid surgery? Eur J Surg Oncol 2017;43:1265-72. [Crossref] [PubMed]
- Brenner RJ, Jackman RJ, Parker SH, et al. Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary? AJR Am J Roentgenol 2002;179:1179-84. [Crossref] [PubMed]
- Consistent protocols for radial scars. Breast Screen Australia. Australian Government Department of Health [published 22 October 2019, accessed 10 July 2020]. Available online: http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/cac-man-rad-scar
- Sohn VY, Causey MW, Steel SR, et al. The treatment of radial scars in the modern era—surgical excision is not required. Am Surg 2010;76:522-5. [Crossref] [PubMed]
- Pinder SE, Shaaban A, Deb R, et al. NHS breast screening multidisciplinary working group guidelines for the diagnosis and management of breast lesions of uncertain malignant potential on core biopsy (B3 lesions). Clin Radiol 2018;73:682-92. [Crossref] [PubMed]
- López-Medina A, Cintora E, Mugica B, et al. Radial scars diagnosed at stereotactic core-needle biopsy: surgical biopsy findings. Eur Radiol 2006;16:1803-10. [Crossref] [PubMed]
- D’orsi C, Bassett L, Berg W, et al. Breast imaging reporting and data system: ACR BI-RADS—breast imaging Atlas. In: D’Orsi C, Mendelson E, Ikeda D, et al. editors. Reston: American College of Radiology, 2003:7-201.
- Resetkova E, Edelweiss M, Albarracin CT, et al. Management of radial sclerosing lesions of the breast diagnosed using percutaneous vacuum-assisted core needle biopsy: recommendations for excision based on seven years’ of experience at a single institution. Breast Cancer Res Treat 2011;127:335-43. [Crossref] [PubMed]
- Lee KA, Zuley ML, Chivukula M, et al. Risk of malignancy when microscopic radial scars and microscopic papillomas are found at percutaneous biopsy. AJR Am J Roentgenol 2012;198:W141-5 [Crossref] [PubMed]
- Breast Cancer Risk Reduction. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology [published 11 December 2018, accessed 10 July 2020]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast_risk_blocks.pdf
- Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology [published 17 May 2019, accessed 10 July 2020]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf
Cite this article as: Bao JJ, Jaskowiak NT. Clinical management of radial scar without atypia diagnosed on core needle biopsy. Ann Breast Surg 2021;5:6.


