
Delay techniques for nipple-sparing mastectomy: a retrospective review of outcomes in 47 patients
Introduction
Nipple-sparing mastectomy (NSM) has become a popular technique of breast cancer surgery when oncologically feasible. Studies have shown that women are more satisfied with the results of breast reconstruction surgery if their own nipple and areola are able to be preserved, as they feel that their reconstructed breasts are more like their own (1,2). In NSM, the breast surgeon is tasked with removing the entire breast, while keeping both the skin flaps and the nipple-areolar complex (NAC) from becoming too thin and devascularized. Necrosis of the soft tissue envelope surrounding a breast prosthesis could lead to revision procedures and ultimately device explantation.
The reported risk of NAC necrosis is between 7–17% (3,4). Risk factors that have described leading to the development of NAC necrosis include ptosis, periareolar scars, high body mass index (BMI), radiation therapy and smoking (4,5). In order to attempt to preserve vascular supply to the nipple, a surgical delay procedure can be performed in the weeks prior to NSM. This is typically performed as an outpatient and can be coupled with sentinel lymph node biopsy and retroareolar biopsy. By performing a retroareolar biopsy in the weeks prior to mastectomy, the decision of whether to preserve the nipple can be based off of permanent pathology results rather than frozen section which has a false negative rate of up to 15% (6).
Many delay techniques have been described for NSM, with various incision types and methods of flap undermining. This article aims to describe a single author’s (K.T.N.) technique for delay prior to mastectomy, along with a retrospective review of the patient demographics, risk factors, and associated outcomes. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/abs-20-61).
Methods
A retrospective review of a single-surgeon’s (K.T.N.) patients who underwent a delay procedure prior to NSM from 2012 to 2017 was performed. Patient demographics were collected, along with history of chemotherapy and radiation, current or previous history of smoking, history of previous breast surgeries and incision location, presence of comorbidities, body mass index, and degree of breast ptosis. The timing and technique of surgical delay was recorded, along with whether the delay was combined with other procedures. Surgical outcomes, including any complications, were also collected. The study was approved by the Institutional Review Board of Rutgers-New Jersey Medical School (IRB Approval Number: XX1102930) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all the patients, providing permission for their medical records and media including photographs to be used for research purposes. Statistical analysis was performed using Microsoft Excel. Categorical data were analyzed by Pearson chi-squared test for independence and continuous data were analyzed by a t-test for comparison of means. A P value of <0.05 was used to determine significance.
The surgical delay procedure was performed by a single surgeon (K.T.N.), typically 7–21 days (mean 15 days) prior to NSM. Incisions were made either in the inframammary fold (Figure 1) or in a lateral curvilinear (Figure 2) or periareolar fashion, with the exception of one patient who had a previous breast incision that was used. Sharp dissection was performed along the chosen incision pattern with a 15-blade, then Bovie electrocautery was used for dissection through the subcutaneous tissues until the breast capsule was visualized. Dissection was then performed in the avascular plane separating the subcutaneous tissues from the breast tissue until the tissues beneath the NAC were completely undermined. At this time, a retroareolar biopsy was performed and sent for permanent pathology. Hemostasis was obtained with Bovie electrocautery and skin was closed in the usual fashion with 3-0 monocryl deep dermal sutures and a 4-0 monocryl subcuticular suture.
Results
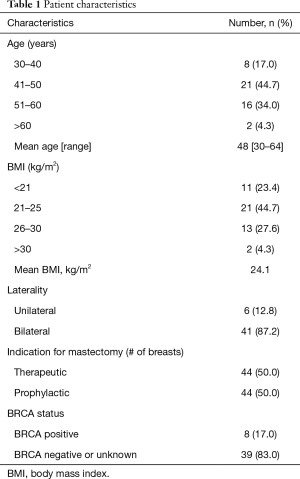
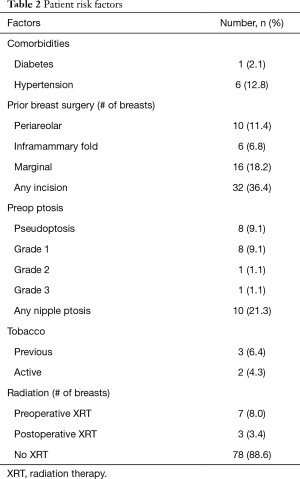
Forty-seven patients (88 breasts) who underwent a delay procedure prior to nipple sparing mastectomy were included in the study. The average age was 48 (range, 30–64) and the average BMI was 24.1. The majority of patients were without significant comorbidities; one patient was diabetic, one patient had a diagnosis of Li Fraumeni syndrome, and six patients had hypertension. Ten patients had ptotic breasts, the majority of these patients had pseudoptosis or grade 1 ptosis. Thirty-two patients had prior breast surgery. Forty-four mastectomies were performed prophylactically, the other 44 mastectomies were performed for biopsy-proven breast cancer. Eight (17%) patients were BRCA positive. Seven patients underwent preoperative radiation therapy, three underwent postoperative radiation. Twelve patients had neoadjuvant chemotherapy and two received adjuvant therapy. Thirty-two patients had previous breast surgeries prior to the delay and mastectomy. There were three patients with history of cigarette smoking, two patients who were current smokers. Patient characteristics and risk factors are listed in Tables 1 and 2, respectively.
Full table
Full table
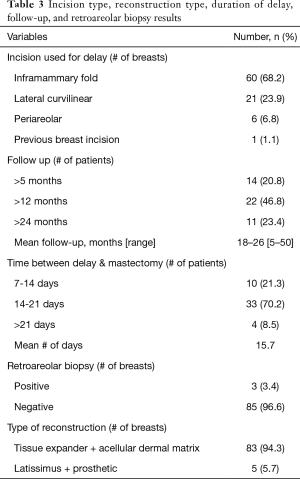
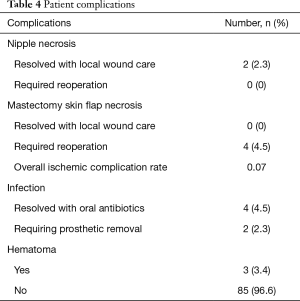
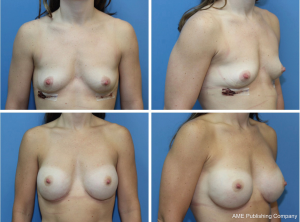
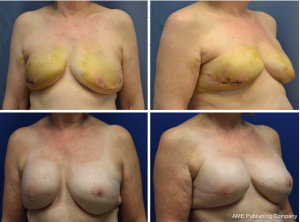
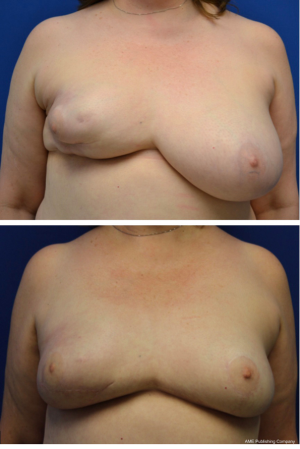
The majority of patients (68.2%) underwent a delay procedure via an inframammary fold incision (Figure 1). Alternatively, a curvilinear incision was used in 23.9% of patients (Figure 2). The average time between delay and NSM was 15.7 days. There were no complications noted after the initial delay procedure. Three patients had a positive retroareolar biopsy and underwent skin-sparing mastectomy. The vast majority of patients underwent implant-based reconstruction with a tissue expander and acellular dermal matrix placed at the time of mastectomy. Five (5.7%) patients had latissimus flap reconstruction with a permanent implant. These results are displayed in Table 3. Post-operatively, two patients had areas of nipple necrosis that resolved without reoperation, four cases of skin necrosis required re-operation. In these patients, three had previous breast surgery and one patient was a smoker. There were three hematomas observed and six incidences of infection, two requiring prosthetic removal. The overall complication rate was 7%, lower than the reported rate in the literature. Complications are listed in Table 4. Three patients who underwent a delay procedure prior to NSM and reconstruction are shown in Figures 3-5. Average follow-up was 13 months (range, 1–48 months).
Full table
Full table
Discussion
Nipple sparing-mastectomy has become a popular choice for women with smaller breasts and peripheral breast lesions and in patients seeking to obtain prophylactic mastectomy who are BRCA positive (7). This option has also become available in patients with larger, more ptotic breasts, as a mastopexy or reduction could be performed in the weeks prior to NSM, allowing for improved positioning of the nipple on the breast mound (8). One of the complications of NSM is partial or complete necrosis of skin flaps and NAC. Studies publish the rates of necrosis around 7–17% (9-11).
As shown in our study, delay procedures prior to mastectomy can allow for improved perfusion to the NAC, decreasing the likelihood of necrosis following NSM. Palmieri was one of the first to describe the use of a delay procedure prior to NSM. As an outpatient under local anesthesia, he described laparoscopically coagulating the deep vascular network beneath the NAC and placing collagen fleece in that region to prevent regeneration of the interrupted blood supply (12). His study also introduced the idea of a subareolar biopsy at the time of delay, allowing for improved detection of cancer extension to the NAC. They observed no NAC necrosis in any of the 18 patients in their series.
There are now several delay techniques described in the literature, shown to be especially useful inpatients deemed “high risk” for NAC necrosis (11). Risk factors that have been associated with NAC necrosis in the literature include previous breast scars, high BMI, significant ptosis, chemotherapy, radiation, and smoking (11,13,14). Tobacco use has been shown to lead to skin flap necrosis in both implant-based and autologous breast reconstruction procedures. Olson showed that even in patients who had quit smoking two weeks prior to surgery, tobacco use was associated with an increased risk of infection (10). In our patient population, one patient who was a smoker five years prior to surgery had incisional breakdown and skin flap necrosis.
In addition to Palmieri’s procedure previously described, there have been various other delay techniques described in the literature. Jensen described radial undermining 5 cm from the nipple circumferentially 3 weeks prior to NSM; no patients in his study had partial or total NAC loss (4). Martinez performs delay procedures via an IMF incision with cephalad undermining around the NAC. He also advocates placement of a silicone sheet in the pocket to prevent revascularization (13). In another study by Martinovic, patients underwent delay via previously made breast scars if possible; otherwise delay incisions were made at the inframammary fold, superolateral or inferolateral to the NAC, or lateral radial. No patients in the study had significant nipple loss and there were only a few cases of epidermolysis that resolved with local wound care (11).
There have been a number of studies showing the utility of a delay procedure in allowing for improved vascularity in patients with ptosis. Spear et al. described the use of a preoperative mastopexy or reduction to allow for improved NAC positioning prior to NSM (8). Schwartz described his results in four patients with ptotic breasts who underwent a delay procedure in the weeks prior to NSM. In their series, they performing a supra-areolar delay incision and undermining down to the inframammary fold, creating an inferior pedicled NAC that is able to be easily maneuvered into an appropriate anatomic position during mastectomy (15). One of the four patients in their series had a small area of nipple necrosis that was treated conservatively, the remainder had no complications.
One of the presumed risk factors for NAC necrosis in NSM is the presence of prior incisions in the periareolar region. Removing the underlying breast tissue forces the NAC to rely solely on the subdermal plexus for perfusion; scars close the nipple can disrupt this dermal blood supply and lead to necrosis. Olson et al. recently published an article regarding whether NSM was safe in patients with prior breast scars. Their study showed no association between scar location and postoperative infection, skin flap necrosis, or NAC necrosis (10).
NSM in our study was performed both in patients with a confirmed breast cancer diagnosis and also prophylactically in patients who were diagnosed as BRCA positive. It has been shown that more aggressive malignant cancer types were not associated with increased incidence of NAC necrosis (16). In our study, cancer stage did not affect outcomes in patients who underwent NSM.
It is known that radiation can poorly affect outcomes of all types of breast reconstruction, as it can impair wound healing and cause lasting effects on the microcirculation of the tissues. In some studies, operative revision rates and complications often leading to explantation have been shown to be higher in patients undergoing radiation therapy (17). However, there are limited studies on the effects of radiation on the NAC. Alperovich described the results of radiation prior to NSM on 26 patients. Their study only showed one incidence of partial NAC necrosis and one incidence of complete NAC necrosis, with no significant difference between irradiated and non-irradiated breasts (18). A meta-analysis published by Zheng et al. in 2017 showed no difference in the odds of NAC necrosis in patients receiving radiation therapy; their study did show, however, a higher likelihood of skin flap necrosis in the patients receiving radiation (19).
Chemotherapy is often used in aggressive breast cancer types as an adjunct treatment with surgery and radiation therapy. Neoadjuvant chemotherapy has been associated with an increased risk of NAC necrosis and implant explantation; this risk is increased when combined with adjuvant therapy (10,20). Chemotherapeutic drugs tend to impair wound healing, and it can be elucidated that this effect leads to poorer outcomes and greater likelihood of ischemic complications.
The importance of NSM for psychosocial well-being of women either diagnosed with breast cancer or with a strong genetic predisposition cannot be underestimated. Studies have shown that level of satisfaction with breasts, body image, and sexual functioning is improved in patients who retain their native NAC during mastectomy (2). While certain limitations do exist for our study, as it is a retrospective review of the outcomes of a single-surgeon, our study shows that the use of a delay procedure prior to NSM, especially in high risk patients, can benefit women by decreasing the likelihood of reoperation for NAC loss or explantation. Further research to continue to improve delay techniques in high risk patients should be performed.
Acknowledgments
The abstract of this manuscript was presented in 2017 at the Annual Meeting of the American Society of Plastic Surgeons and its abstract was accordingly published in Plastic and Reconstructive Surgery Global Open. This completed manuscript has not been published in any other form elsewhere and is not under consideration by any other journal.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-61
Data Sharing Statement: Available at http://dx.doi.org/10.21037/abs-20-61
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-61). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Rutgers-New Jersey Medical School (IRB Approval Number: XX1102930) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all the patients, providing permission for their medical records and media including photographs to be used for research purposes.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Satteson ES, Brown NJ, Nahabedian MY. Nipple-areolar complex reconstruction and patient satisfaction: a systematic review and meta-analysis. Gland Surg 2017;6:4-13. [Crossref] [PubMed]
- Metcalfe KA, Cil TD, Semple JL, et al. Long-Term Psychosocial Functioning in Women with Bilateral Prophylactic Mastectomy: Does Preservation of the Nipple-Areolar Complex Make a Difference. Ann Surg Oncol 2015;22:3324-30. [Crossref] [PubMed]
- Karian LS, Therattil PJ, Wey PD, et al. Delay Techniques for Nipple-Sparing Mastectomy: A Systematic Review. J Plast Reconstr Aesthet Surg 2017;70:236-42. [Crossref] [PubMed]
- Jensen JA, Lin JH, Kapoor N, et al. Surgical delay of the nipple areolar complex: a powerful technique to maximize nipple viability following nipple-sparing mastectomy. Ann Surg Oncol 2012;19:3171-6. [Crossref] [PubMed]
- Cho JW, Yoon ES, Kim HS, et al. Nipple-Areola Complex Necrosis after Nipple-Sparing Mastectomy with Immediate Autologous Breast Reconstruction. Arch Plast Surg 2015;42:601-7. [Crossref] [PubMed]
- Luo D, Ha J, Latham B, et al. The accuracy of intraoperative subareolar frozen section in nipple-sparing mastectomies. Ochsner J 2010;10:188-92. [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [Crossref] [PubMed]
- Spear SL, Rottman SJ, Seiboth LA, et al. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg 2012;129:572-81. [Crossref] [PubMed]
- Orzalesi L, Casella D, Santi C, et al. Nipple sparing mastectomy: surgical and oncologic outcomes from a national multicentric registry with 913 patients (1006 cases) over a six year period. Breast 2016;25:75-81. [Crossref] [PubMed]
- Olson J, Anderson LA, Ying J, et al. Nipple Sparing Mastectomy in Patients with Prior Breast Scars. Ann Plast Surg 2017;78:22-7. [Crossref] [PubMed]
- Martinovic ME, Pellicane JV, Blanchet NP. Surgical delay of the nipple-areolar complex in high-risk nipple sparing mastectomy reconstruction. Plast Reconstr Surg Glob Open 2016;4:e760 [Crossref] [PubMed]
- Palmieri B, Baitchev G, Grappolini S, et al. Delayed nipple-sparing modified subcutaneous mastectomy: rationale and technique. Breast J 2005;11:173-8. [Crossref] [PubMed]
- Martinez CA, Reis SM, Boutros SG. The nipple areola preserving mastectomy: the value of adding a delay procedure. Plast Reconstr Surg Glob Open 2016;4:e1098 [Crossref] [PubMed]
- Bertoni DM, Nguyen D, Rochlin D, et al. Protecting nipple perfusion by devascularization and surgical delay in patients at risk for ischemic complications during nipple-sparing mastectomies. Ann Surg Oncol 2016;23:2665-72. [Crossref] [PubMed]
- Schwartz JC, Skowronksi PP. Surgical Delay Facilitates Pedicled Nipple-sparing Mastectomy and Reconstruction in the Ptotic Patient. Plast Reconstr Surg Glob Open 2016;4:e735 [Crossref] [PubMed]
- Lohsiriwat V, Rottmenzs N, Botteri E, et al. Do clinicopathological features of the cancer patient relate with nipple areolar complex necrosis in nipple-sparing mastectomy? Ann Surg Oncol 2013;20:990-6. [Crossref] [PubMed]
- Reish RG, Lin A, Phillips NA, et al. Breast reconstruction outcomes after nipple-sparing mastectomy and radiation therapy. Plast Reconstr Surg 2015;135:959-66. [Crossref] [PubMed]
- Alperovich M, Choi M, Frey JD, et al. Nipple-Sparing Mastectomy in Patients with Prior Breast Irradiation: Are Patients at Higher Risk for Reconstructive Complications? Plast Reconstr Surg 2014;134:202e-206e. [Crossref] [PubMed]
- Zheng Y, Zhong M, Ni C, et al. Radiotherapy and nipple-areolar complex necrosis after nipple-sparing mastectomy: a systematic review and meta-analysis. Radiol Med 2017;122:171-8. [Crossref] [PubMed]
- Frey JD, Choi M, Karp NS. The effect of neoadjuvant chemotherapy compared to adjuvant chemotherapy in healing after nipple-sparing mastectomy. Plast Reconstr Surg 2017;139:10e-19e. [Crossref] [PubMed]
Cite this article as: Halsey JN, Chandler LK, Wey PD, Nini KT. Delay techniques for nipple-sparing mastectomy: a retrospective review of outcomes in 47 patients. Ann Breast Surg 2021;5:3.



