
Vascularized lymph vessel transplant (VLVT): our experience and lymphedema treatment algorithm
Introduction
The surgical treatment of lymphedema has become a hot topic in the field of reconstructive surgery. Advancements over the past few decades have given rise to a myriad of efficacious options for these patients. While lymphedema cure is now a clinical reality (1), there still is no consensus on the management of this chronic, morbid condition. Supermicrosurgical lymphaticovenicular anastomosis (LVA) and vascularized lymph node transplant (VLNT) are established “physiologic” techniques for the treatment of fluid-predominant lymphedema (2). Because it requires functioning lymphatics, the success rate of LVA is significantly higher in early-stage disease (2,3). VLNT is traditionally indicated for more advanced disease (2,4). While VLNT is effective reducing limb volume and the associated morbidity, iatrogenic donor-site lymphedema is an ever-present possibility. The potential to cause the very disease this procedure is intended to treat may be viewed as an unacceptable risk. The appeal of this procedure is further diminished by contour deformities in the recipient limb caused by the bulky flap (2,4).
VLNT is theorized to work through “bridge” and “pump” mechanisms. The former proposes that transplanted nodes stimulate lymphangiogenesis via VEGF, connecting them with recipient-site lymphatic vessels (5-7). The latter posits that arteriovenous perfusion gradients allow the transplanted nodes to act as sponges or pumps, drawing lymph into the circulation through established lymphovenous connections in the transferred tissues (8-10). However, increasing experience with VLNT strongly suggests that the active therapeutic component of VLNT is the lymphatic vessels, not the nodes themselves (11-17). It has long been observed that standard free tissue transfer without nodal tissue can incidentally improve or restore lymphatic outflow of recipient sites (18-20)—a phenomenon thought to be due to a lymph-vessel-stimulated bridge mechanism (21). Similarly, nodes are not thought to be the major players in the collection and pumping of lymph; while the endothelial cells lining intranodal channels do have contractile function, the smooth muscles lining the transplanted lymph vessels are the main drivers of lymph transport (22-27). This new understanding has raised the possibility of restoration of lymphatic outflow without lymphatic anastomosis or lymph node (LN) transplant, giving rise to a new addition to the armamentarium for treating extremity lymphedema: vascularized lymph vessel transplant (VLVT).
Diagnostic evaluation
Classically, diagnosis of lymphedema has been clinical, relying on the Kaposi-Stemmer sign (inability to pinch skin of the affected extremity) and a significant increase in limb volume from baseline (while there is no universal consensus on this criteria, a 10% increase is most commonly used). Volumetric changes may be measured via three-dimensional (3D) optoelectronic perometry, water displacement, 3D volumetric scanning with an iPad app, or volume calculations derived from circumference measurements (Figure 1). Other often-discussed signs are swelling that does not respond appropriately to elevation or diuretics, blunted, squared-off digits, and skin changes (2,28-31). However, the refinement of diagnostic imaging has revealed that these clinical features have poor sensitivity, positive and negative predictive value, and accuracy (28,32). Venous insufficiency, congestive heart failure, lipedema, renal failure, and venous thrombosis, to name a few, may all exhibit these “classic” clinical signs (28,30,31). Another flaw to this diagnostic strategy is that volumetric measurement relies on the assumption that disease severity correlates with limb volume—in intermediate-to-advanced lymphedema, a solid disease component develops and this assumption no longer holds true (32). Several qualitative questionnaires assessing lymphedema symptomatology and its impact on quality of life (QoL) exist. While they similarly are not sufficient for definitive diagnosis, they serve as a key component of a comprehensive diagnostic evaluation (32-35).
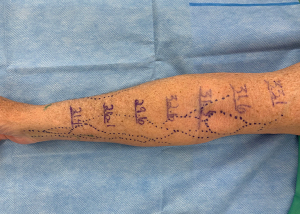
Anecdotally, our practice frequently encounters patients who have been diagnosed with these aforementioned techniques, without any proper confirmatory study. Imaging to confirm the diagnosis of lymphedema and stratify disease severity is imperative. Indocyanine green (ICG) lymphography is quickly replacing lymphoscintigraphy as the gold standard in lymphedema diagnosis (2,32,36). This technique measures lymph vessel pump function, velocity, and distribution in real time (32,37). Because it can visualize lymphatic function and dermal backflow patterns, it can effectively stage disease (Figure 2) (32,36,38). Following definitive diagnosis and staging with ICG lymphography, adjunctive modalities can further define the disease state and determine fluid- or solid-predominance (39). Quantitative bioimpedance spectroscopy (BIS) is a painless, cost-effective technique to determine the amount of extracellular fluid in an extremity (29,31). Magnetic resonance imaging (MRI) can differentiate fluid from fibrosis and lipodystrophy, the structural components of solid-predominant lymphedema (29).
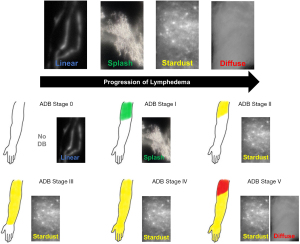
There is no consensus on the quantitative threshold of tissue bulk that distinguishes these two states. Qualitatively, solid-predominance is a state in which bulky lipodystrophy and fibrosis have become the main drivers of morbidity. Physical exam will show little-to-no pitting and these patients will not experience significant volume reduction even after aggressive complex decongestive therapy (CDT) (40). MRI will demonstrate significant lipodystrophy and/or subcutaneous fibrosis (2,41). In contrast, fluid-predominant lymphedema is demonstrated by prominent pitting on physical examination, minimal lipodystrophy and fibrosis on MRI, and significant limb fluid excess on BIS. It is noted to demonstrate dramatic (if temporary) volume reduction in response to CDT, even in advanced disease (31,32,41).
Conventional surgical treatment options
Vascularized lymph node transplant
Traditionally, the superficial circumflex iliac artery (SCIA)-based groin flap served as the workhorse of VLNT, due to reliable anatomy, abundant LNs, and well-hidden scar (9,30,42,43). Overall complication rates are low; reported adverse effects include infection, delayed wound healing, lymphorrhea, reexploration, flap loss, and contour deformity requiring secondary debulking procedures (44-46). Iatrogenic donor-site lymphedema had long been discussed as a potential complication (47-49), but it remained theoretical until 2013, when two independent reports of this complication were published (50,51). The potential for this devastating complication spurred an explosion of alternative VLNT flaps, including the submental (52-54), supaclavicular (55,56), lateral thoracic (57,58), omental (59,60), and jejunal (13,61) flaps. While these carry a lower risk of donor-site lymphedema (30,45,58,62), each carries its own set of disadvantages: marginal mandibular nerve damage (submental), spinal accessory or phrenic nerve injury (supraclavicular), intercostobrachial nerve injury (lateral thoracic), and violation of the peritoneum or bowel ischemia (omental, jejunal) (3,13,54,57-60,63).
Supermicrosurgical lymphaticovenular anastomosis
The advent of supermicrosurgery has facilitated LVA with small, low-pressure venules ranging from 0.1–0.6 mm (64). The use of smaller vessels allows for anastomosis in the distal extremities, circumventing the degradation of lymph vessels’ pumping function, which begins proximally (65). A number of anastomotic configurations are available to overcome unfavorable pressure gradients, vessel size mismatch, difficult vessel positions, or vessel number mismatch. Named in a lymphatic-to-vein convention, these include end-to-end, end-to-side, side-to-side, side-to-end, lambda, double end-to-side, ladder, and “octopus” anastomosis (Figure 3) (31,66,67). While LVA is efficacious, it requires supermicrosurgical technique and costly equipment not available at all centers (2-4,66). Post-radiotherapy fibrosis and post-surgical perivascular scarring must be minimal; otherwise, resultant venous hypertension could still cause reflux into the anastomosed lymphatic channels, worsening lymphedema over time (2,3,30). Intra-operative ICG is required following anastomosis in order to confirm patency and flow (2). An additional barrier to entry is the wide heterogeneity of patient selection, timing, and number, location, and configuration of anastomoses (30,64,65).
Vascularized lymph vessel transplant
Background
LVA and VLNT dominated the field until Koshima et al.’s seminal 2016 paper describing the successful management of severe lower extremity lymphedema with a lymphadiposal flap based on the first dorsal metatarsal artery (FDMA) (68). This novel approach challenged the accepted belief that treatment of lymphedema requires the incorporation of cumbersome LNs into free flaps (4). Advancements in lymphography now facilitate precise pre- and intraoperative visualization of superficial lymph vessels, facilitating intentional inclusion in flaps and proper orientation in recipient sites (19). The senior author experienced favorable outcomes with FDMA-based VLVT (4). However, the location of the donor site precluded the flap’s use in bilateral lower extremity disease. Additionally, FDMA flap harvest frequently caused devascularization of the skin over the first metatarsal space, resulting in donor wound breakdown (4). Nevertheless, the success of the procedure encouraged us to search for alternative donor sites for VLVT.
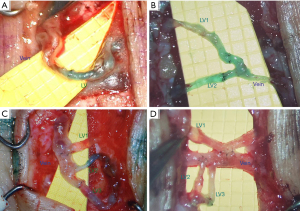
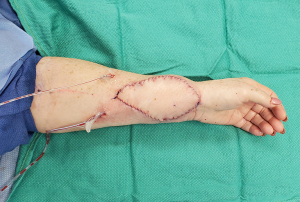
The SCIA perforator (SCIP) flap’s role in groin flap-based VLNT makes its anatomy familiar to plastic surgeons. It has a high density of superficial lymph vessels and an excellent track record as a superthin flap. The distinct visual difference between the small superficial fat lobules and large deep fat lobules makes for an easy plane of dissection. This technique allows for the preservation of LNs and other deep structures, reducing the risk for complications such as lymphorrhea and wound dehiscence. Results of SCIP flap-based VLVT show highly favorable outcomes without the contour deformity associated with classic groin flap-based VLNT (Figure 4) (4,31,69-75). Due to its superior outcomes, SCIP-based VLVT replaced VLNT as our go-to treatment for those with upper extremity lymphedema who are not LVA candidates. Following the success of SCIP-based VLVT, we applied the same principle to develop thoracodorsal artery perforator (TDAP)-based VLVT to treat lower extremity lymphedema. The lateral thoracic region is another area rich with lymphatic vessels (58). By performing a thin flap harvest superficial to the superficial fascia, lateral thoracic LNs are preserved. Our results with this technique have shown similar efficacy to SCIP-based VLVT with minimal donor-site morbidity.
Pre-operative evaluation
Vascular anatomy can be imaged preoperatively with computed tomography angiography (CTA), allowing the more robust-appearing side to be chosen for harvest. Preoperative vascular imaging can be especially helpful for TDAP flaps, as TDAP vascular anatomy and perforator location is more variable than that of the SCIP flap. It is, however, not uncommon for CTA to miss small perforators (<0.3 mm) that are subsequently found upon surgical exploration. Thus, a negative CTA is not an absolute contraindication to perforator flap harvest (Video 1). However, when vessels are detected on CTA, this can expedite intra-operative surgical planning and dissection; therefore, in our experience, it is still worthwhile to routinely pursue pre-operative imaging, despite its limitations. Duplex ultrasound, particularly high-resolution duplex ultrasound, is a powerful alternative modality that can detect vessels as small as 0.18 mm. However, it is limited by a steep learning curve and operator dependency (76-78).
Surgical technique
SCIP flap
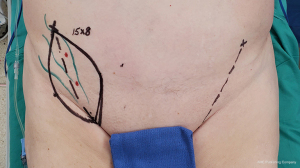
0.25% ICG is injected intradermally lateral to the anterior superior iliac spine (ASIS) to map superficial groin lymphatic vessels. A line from the groin crease to the ASIS is marked, and SCIPs are identified along this line with Doppler ultrasound. Flap width is determined with a pinch test to ensure the ability to primarily close the donor site, and a SCIP flap is designed over the perforators and mapped lymphatics (Figure 5). The inferolateral incision is made, allowing for cephalad-directed, lateral-to-medial dissection. The dissection plane is immediately superficial to Scarpa’s fascia, ensuring exclusion of LNs from the flap. After confirming perforator entry into the flap, the superomedial skin incision is made and retrograde pedicle dissection is performed until adequate pedicle length is achieved. The adiposal surface of the flap is scanned with SPY Elite (Stryker Corporation, Kalamazoo, MI, USA) to confirm the presence of lymphatic vessels in the flap. Following flap harvest, the donor site is closed primarily in a layered fashion (4).
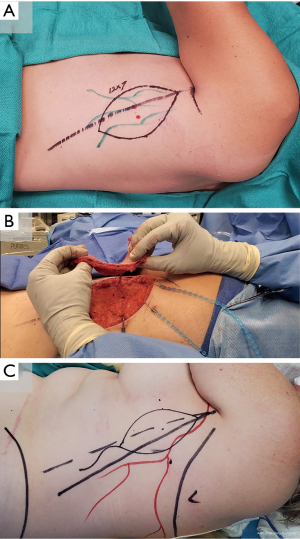
TDAP flap
Lymphatics are mapped with intradermal ICG injection of the fifth intercostal space at the midaxillary line. Perforators are identified with Doppler ultrasonography 8–10 cm inferior to the axillary apex and 1–2 cm inside the lateral border of the latissimus dorsi. A flap is designed around the perforators and marked lymphatics (Figure 6). The posterior incision is made and dissection proceeds posteriorly along the superficial fascial plane. The committing anterior incision is made after confirming perforator location. Following flap harvest, the donor site is closed primarily in a layered fashion (79-81).
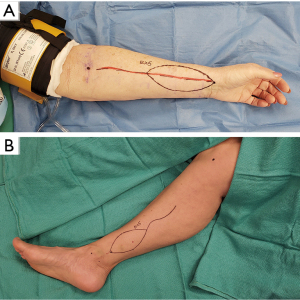
Vascular anastomosis and flap inset
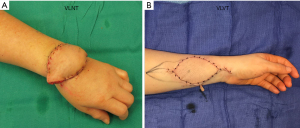
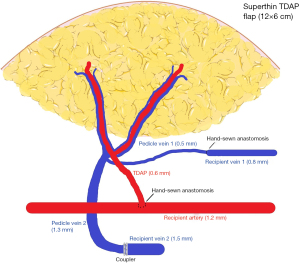
The VLVT flap is inset distally. In order for lymphangiogenesis to effectively “bridge” between the flap and recipient site, the direction of lymph flow in the flap must be concordant with that of the native lymphatics. Thus, the flap must be oriented so that the axiality of lymphatic vessels is compatible (19). In the upper extremity, the radial aspect of the wrist is used with the radial artery as the recipient vessel. In the lower extremity, it is inset along the medial aspect of the lower leg using the posterior tibial artery as a recipient (Figure 7). The flap arterial pedicle is often smaller than 0.8 mm, necessitating supermicrosurgical technique. When a suitable recipient perforator is present, end-to-end perforator-to-perforator anastomosis is performed. However, recipient vessels are often too large, which would result in excessive arterial inflow. Surgeons should therefore anticipate the need to perform end-to-side anastomosis. End-to-end venous anastomosis is performed between the flap venous pedicle and a recipient pedicle venae comitante. It is often possible to use venous couplers, but surgeons should be prepared to perform suture anastomosis in the event of small vein size or size mismatch (Figure 8). In keeping with the principles of VLVT and VLNT, no lymphatico-venicular or lymphatico-lymphatical anastomoses are performed. Following revascularization, a full-thickness skin excision with dimensions identical to the VLVT flap is made to facilitate inset.
Postoperative care
The recipient limb is elevated for 1 week postoperatively. A progressive, graduated bandage compression protocol is then initiated in the second postoperative week; patients are rapidly advanced from brief compression to 16 hours per day. One month postoperatively, patients re-commence CDT, including transitioning into newly fitted 30–40 mmHg compression garments for 16 hours per day. Weaning of compressive garments may begin at 6 months postoperatively. Postoperative surveillance is conducted via the same set of diagnostic studies performed preoperatively, allowing comparison to patient baselines. In the senior author’s practice, this consists of ICG lymphography to confirm and monitor improvement, BIS to evaluate fluid and solid disease components, 3D volumetric scanning, and a lymphedema-specific QoL assessment (32) to track symptomatology. These are performed at 3, 6, 12 months, and then annually to track patient progress.
Pitfalls and considerations
The only notable VLVT complication thus far in the senior author’s practice has been one case of partial (<5%) flap loss that was successfully managed with wound care.
For VLVT, we advocate for either the SCIP or the TDAP flap, due to the aforementioned limitations of the FDMA flap. Regardless of the flap chosen, harvest of a superthin flap likely requires the use of perforator vessels and consequently, supermicrosurgical technique (81,82). Surgeons must be aware that TDAP perforators frequently originate from an intercostal or lateral thoracic vessel, rather than from the thoracodorsal. This considerable anatomic variability, in combination with the previously discussed limitations of CTA, can lead to increased difficulty when utilizing the TDAP flap. However, provided that perforators can be located and adequate pedicle length can be achieved, harvesting this flap on these alternative vessels is still feasible (80,81).
While Scarpa’s fascia facilitates ease of SCIP flap dissection in patients of normal BMI, using this plane in obese patients results in an excessively thick flap requiring secondary debulking surgery (Figure 9) (72). Previously, the senior author circumvented this by raising the flap along a non-anatomic plane superficial to the superficial fascia 5 mm deep to the skin (4,74). However, with further experience, we have abandoned this technique because it does not consistently include the superficial circumflex iliac vein. We now recommend adhering to the plane immediately superficial to the superficial fascia as the plane of dissection because it is safe and easier. The TDAP can be similarly bulky in obese patients. Defatting after flap elevation is feasible, but tedious (79). In general, the senior author prefers to use SCIP-based VLVT due to the flap’s more reliable anatomy. In those cases where it is unavailable (i.e., in patients with gynecological cancer or compromised lower extremity lymphatics), TDAP is chosen.
Lymphedema management algorithm and discussion
Treatment selection is guided by the severity of injury seen on ICG combined with individual patient needs. Generally, one should offer the least invasive procedure that will still offer satisfactory outcomes (Figure 10) (2-4,32,83-85). CDT, traditionally considered first-line, has not been shown to cure or fundamentally impact the disease course. Surgery, which can halt, or in select cases, even reverse progression, is typically offered to patients who have failed therapy (3,30,40). However, with no standardization of CDT, it is difficult to define what constitutes an adequate trial or treatment failure. The rigor of lymphedema therapy is another complicating factor—success requires lifelong commitment to a strict regimen (2). We therefore discourage the categorization of CDT as first-line and surgery as second-line; with careful patient selection, both are effective options. In younger patients or in patients with severe disease who cannot tolerate the rigor of CDT, early initiation of an aggressive surgical plan may be appropriate, even if they have only trialed therapy for a short time. In patients with early disease, providers have more time to adjust CDT to their tolerance.
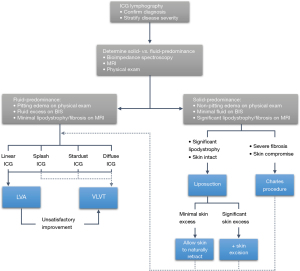
Physiologic procedures such as LVA, VLNT, and now, VLVT restore drainage of the affected limb. Thus, they are most appropriate for fluid-predominant lymphedema (12,41). To date, no direct comparisons of VLNT and VLVT have been published. However, in the experience of the senior author and his practice, they perform identically. One must speculate, then—do LNs truly serve a purpose in VLNT? In practice, there is no consistent evidence different LN flaps—which often contain different numbers of nodes—produce different outcomes (12,14,15). The sole study comparing different VLNT flaps within a single institution did not demonstrate a significant difference (16). More controlled efforts to establish a correlation between the number of LNs and the effectiveness of the flap have yielded mixed results (13,17). It is difficult to compare VLNT flaps with and without skin paddles in a controlled manner, but an increasing understanding of the dermal and subcutaneous lymphatic system strongly suggests that this lymphadiposal tissue is essential for efficacy of the flap (11,86). This, combined with lymphatic channels’ abilities to absorb lymph and stimulate lymphangiogenesis, supports the senior author’s argument that vessels—not nodes—are the therapeutic components of these flaps (18-24). Due to the novel nature of VLVT, long-term outcome data is limited; further follow-up is warranted to characterize long-term efficacy and sequelae. To date, the senior author has demonstrated highly favorable reduction of lymphedema volume and symptoms with a minimal rate of morbidity. After this group’s initial publication on VLVT in 2019 (4), the senior author has since had a select number of patients discontinue the wearing of compression garments entirely. These outcomes have led the senior author’s practice to phase VLNT out in favor of VLVT.
Supermicrosurgical LVA comes with a steep learning curve. While it is conceptually simple, the nuances of this procedure take extensive experience to master. There is much discussion in the literature about technical competency in supermicrosurgical anastomosis. However, achieving favorable results requires more than this. Understanding lymphatic and venous physiology and pathophysiology is imperative for proper selection of surgical sites and vessels (87). These obstacles are daunting, but in the senior author’s opinion, mastering LVA is a worthwhile pursuit for beginning surgeons; in experienced hands, it is an incredibly powerful procedure.
There is no clear indication for when surgeons should stop offering LVA in favor of VLVT. For beginning surgeons, we recommend a black-and-white approach. If an abundance of linear ICG patterns are present, LVA is expected to be technically straightforward. VLVT should be performed in the absence of linear patterns as this indicates a paucity of available healthy lymphatics. Advanced lymphedema surgeons may adopt a more nuanced perspective, incorporating individual judgment and patient expectations. Functioning lymphatic vessels can frequently be found upon intraoperative search with high-resolution duplex ultrasound in patients with no linear ICG patterns. If these vessels can be anastomosed, LVA can remain feasible and effective even in patients with severe disease (88,89). Because one cannot make evidence-based recommendations on which procedure is more appropriate, providers should actively involve patients in the decision-making process. Preoperative discussions should include both LVA and VLVT, with the possible exception of those early cases where LVA is the clear-cut recommendation. The choice often hinges on the degrees of morbidity and recovery the patient is willing to accept. For severe disease treated with LVA, patients must be counseled that escalation to VLVT may be necessary should LVA be unsuccessful.
LVA and VLNT can be performed simultaneously in a “cocktail” approach. Proponents argue that they provide synergistic benefits—we do not share this opinion (85). LVA and VLNT have contrasting indications and should ideally not be combined. In lymphedema severe enough to require VLNT, there is significant lymphatic injury with poor-quality channels. Because LVA requires functioning lymph vessels to be successful, it is not expected to work in these cases. In contrast, in patients selected for LVA, LVA should likely be sufficient by itself without the addition of VLNT (41). Should surgeons desire to explore this “cocktail” approach, LVA combined with VLVT—a less morbid procedure—may be appropriate.
Patients with a significant solid disease component should be offered non-physiologic procedures such as liposuction, which can effect powerful volume and symptom reduction in patients with late-stage lymphedema (2,40,90,91). Traditionally, when staging liposuction and physiologic treatment, most surgeons perform physiologic procedures first, perhaps due to early concerns that liposuction could further damage lymphatics or worsen the progression of disease. However, liposuction has not been found to cause injury; on the contrary, it triggers lymphatic regeneration, improving drainage on lymphoscintigraphic and ICG lymphographic studies (40,92-95). Moreover, when performing liposuction second, surgeons must either avoid their anastomoses, thereby potentially undertreating the patient, or risk ruining their reconstruction. Staging LVA or VLNT following liposuction has been shown to improve outcomes—removing lipodystrophy and improving drainage can set the stage for a more effective reconstruction (91,96). To date, the senior author has performed VLVT following liposuction in one patient, with highly favorable results. The excess skin created following liposuction is traditionally allowed to contract over time. However, it has been shown that concurrent skin excision is safe and effective (Video 2, Figure 11) (40,97). Skin excision may, on occasion, reveal fibrotic fascia with a cement-like appearance. A concurrent fascial release may be appropriate to mitigate the theoretical risk of chronic compartment syndrome.
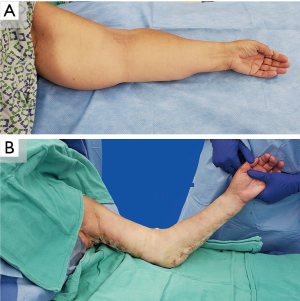
Conclusions
Surgical treatment of extremity lymphedema effects clinically significant improvements in symptomatology and QoL. VLVT based on the SCIP flap and now, the TDAP flap, presents a promising alternative to VLNT. Further research is warranted to understand long-term outcomes and refine patient selection.
Acknowledgments
The authors would like to thank Erika Hopkins for her assistance with providing photographs for the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dung Nguyen) for the series “Cutting-edge of Complex Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-20-139/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-20-139/coif). The series “Cutting-edge of Complex Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen WF, McNurlen M, Bowen M. Surgical treatments of lymphedema. In: Jones GE. editor. Bostwick’s plastic and reconstructive breast surgery. 4th ed. New York: Thieme Medical Publishers, 2020:1478-99.
- Gallagher KK, Lopez M, Iles K, et al. Surgical approach to lymphedema reduction. Curr Oncol Rep 2020;22:97. [Crossref] [PubMed]
- Schaverien MV, Coroneos CJ. Surgical treatment of lymphedema. Plast Reconstr Surg 2019;144:738-58. [Crossref] [PubMed]
- Chen WF, Mcnurlen M, Ding J, et al. Vascularized lymph vessel transfer for extremity lymphedema - is transfer of lymph node still necessary? Int Microsurg J 2019;3:1. [Crossref]
- Honkonen KM, Visuri MT, Tervala TV, et al. Lymph node transfer and perinodal lymphatic growth factor treatment for lymphedema. Ann Surg 2013;257:961-7. [Crossref] [PubMed]
- Aschen SZ, Farias-Eisner G, Cuzzone DA, et al. Lymph node transplantation results in spontaneous lymphatic reconnection and restoration of lymphatic flow. Plast Reconstr Surg 2014;133:301-10. [Crossref] [PubMed]
- Hartiala P, Saaristo AM. Growth factor therapy and autologous lymph node transfer in lymphedema. Trends Cardiovasc Med 2010;20:249-53. [Crossref] [PubMed]
- Cheng MH, Huang JJ, Wu CW, et al. The mechanism of vascularized lymph node transfer for lymphedema: natural lymphaticovenous drainage. Plast Reconstr Surg 2014;133:192e-8e. [Crossref] [PubMed]
- Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg 2009;123:1265-75. [Crossref] [PubMed]
- Ito R, Zelken J, Yang CY, et al. Proposed pathway and mechanism of vascularized lymph node flaps. Gynecol Oncol 2016;141:182-8. [Crossref] [PubMed]
- Miranda Garcés M, Pons G, Mirapeix R, et al. Intratissue lymphovenous communications in the mechanism of action of vascularized lymph node transfer. J Surg Oncol 2017;115:27-31. [Crossref] [PubMed]
- Pappalardo M, Patel K, Cheng MH. Vascularized lymph node transfer for treatment of extremity lymphedema: an overview of current controversies regarding donor sites, recipient sites and outcomes. J Surg Oncol 2018;117:1420-31. [Crossref] [PubMed]
- Coriddi M, Wee C, Meyerson J, et al. Vascularized jejunal mesenteric lymph node transfer: a novel surgical treatment for extremity lymphedema. J Am Coll Surg 2017;225:650-7. [Crossref] [PubMed]
- Chang EI, Chu CK, Hanson SE, et al. Comprehensive overview of available donor sites for vascularized lymph node transfer. Plast Reconstr Surg Glob Open 2020;8:e2675. [Crossref] [PubMed]
- Hanson SE, Chang EI, Schaverien MV, et al. Controversies in surgical management of lymphedema. Plast Reconstr Surg Glob Open 2020;8:e2671. [Crossref] [PubMed]
- Ciudad P, Agko M, Perez Coca JJ, et al. Comparison of long-term clinical outcomes among different vascularized lymph node transfers: 6-year experience of a single center’s approach to the treatment of lymphedema. J Surg Oncol 2017;116:671-82. [Crossref] [PubMed]
- Gustafsson J, Chu SY, Chan WH, et al. Correlation between quantity of transferred lymph nodes and outcome in vascularized submental lymph node flap transfer for lower limb lymphedema. Plast Reconstr Surg 2018;142:1056-63. [Crossref] [PubMed]
- Slavin SA, Upton J, Kaplan WD, et al. An investigation of lymphatic function following free-tissue transfer. Plast Reconstr Surg 1997;99:730-41. [Crossref] [PubMed]
- Yamamoto T, Iida T, Yoshimatsu H, et al. Lymph flow restoration after tissue replantation and transfer: Importance of lymph axiality and possibility of lymph flow reconstruction without lymph node transfer or lymphatic anastomosis. Plast Reconstr Surg 2018;142:796-804. [Crossref] [PubMed]
- Khazanchi RK, Rakshit K, Bal CS, et al. Evaluation of lymphatic drainage in free flaps by lymphoscintigraphy: a preliminary study. Br J Plast Surg 1996;49:123-8. [Crossref] [PubMed]
- Yan A, Avraham T, Zampell JC, et al. Mechanisms of lymphatic regeneration after tissue transfer. PLoS One 2011;6:e17201. [Crossref] [PubMed]
- Aukland K, Reed R. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev 1993;73:1-78. [Crossref] [PubMed]
- Schmid-Schonbein GW, Zweifach BW. Fluid pump mechanisms in initial lymphatics. Physiology 1994;9:67-71. [Crossref]
- Scallan JP, Zawieja SD, Castorena-Gonzalez JA, et al. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol 2016;594:5749-68. [Crossref] [PubMed]
- Ikomi F, Kawai Y, Ohhashi T. Recent advance in lymph dynamic analysis in lymphatics and lymph nodes. Ann Vasc Dis 2012;5:258-68. [Crossref] [PubMed]
- O’Melia MJ, Lund AW, Thomas SN. The biophysics of lymphatic transport: engineering tools and immunological consequences. iScience 2019;22:28-43. [Crossref] [PubMed]
- Randolph GJ, Ivanov S, Zinselmeyer BH, et al. The lymphatic system: integral roles in immunity. Annu Rev Immunol 2017;35:31-52. [Crossref] [PubMed]
- Jayaraj A, Raju S, May C, et al. The diagnostic unreliability of classic physical signs of lymphedema. J Vasc Surg Venous Lymphat Disord 2019;7:890-7. [Crossref] [PubMed]
- Grada AA, Phillips TJ. Lymphedema: diagnostic workup and management. J Am Acad Dermatol 2017;77:995-1006. [Crossref] [PubMed]
- Allen RJ, Cheng MHH. Lymphedema surgery: patient selection and an overview of surgical techniques. J Surg Oncol 2016;113:923-31. [Crossref] [PubMed]
- Chen WF, McNurlen M, Bowen M. Surgical treatments of lymphedema. In: Jones GE. editor. Bostwick’s plastic and reconstructive breast surgery. 4th ed. New York: Thieme Medical Publishers, 2020:1478-99.
- Chen WF, Zhao H, Yamamoto T, et al. Indocyanine green lymphographic evidence of surgical efficacy following microsurgical and supermicrosurgical lymphedema reconstructions. J Reconstr Microsurg 2016;32:688-98. [Crossref] [PubMed]
- Patel KM, Lin CY, Cheng MH. A prospective evaluation of lymphedema-specific quality-of-life outcomes following vascularized lymph node transfer. Ann Surg Oncol 2015;22:2424-30. [Crossref] [PubMed]
- Brorson H, Ohlin K, Olsson G, et al. Quality of life following liposuction and conservative treatment of arm lymphedema. Lymphology 2006;39:8-25. [PubMed]
- Cormier JN, Xing Y, Zaniletti I, et al. Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology 2009;42:161-75. [PubMed]
- Yamamoto T, Yamamoto N, Doi K, et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: A novel severity staging system using dermal backflow patterns. Plast Reconstr Surg 2011;128:941-7. [Crossref] [PubMed]
- Unno N, Nishiyama M, Suzuki M, et al. Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur J Vasc Endovasc Surg 2008;36:230-6. [Crossref] [PubMed]
- Suami H, Heydon-White A, Mackie H, et al. A new indocyanine green fluorescence lymphography protocol for identification of the lymphatic drainage pathway for patients with breast cancer-related lymphoedema. BMC Cancer 2019;19:985. [Crossref] [PubMed]
- Granzow JW, Soderberg JM, Kaji AH, et al. An effective system of surgical treatment of lymphedema. Ann Surg Oncol 2014;21:1189-94. [Crossref] [PubMed]
- Brorson H. Liposuction in lymphedema treatment. J Reconstr Microsurg 2016;32:56-65. [PubMed]
- Chen WF. How to get started performing supermicrosurgical lymphaticovenular anastomosis to treat lymphedema. Ann Plast Surg 2018;81:S15-20. [Crossref] [PubMed]
- Saaristo AM, Niemi TS, Viitanen TP, et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg 2012;255:468-73. [Crossref] [PubMed]
- Nguyen AT, Chang EI, Suami H, et al. An algorithmic approach to simultaneous vascularized lymph node transfer with microvascular breast reconstruction. Ann Surg Oncol 2015;22:2919-24. [Crossref] [PubMed]
- Basta MN, Gao LL, Wu LC. Operative treatment of peripheral lymphedema: a systematic meta-analysis of the efficacy and safety of lymphovenous microsurgery and tissue transplantation. Plast Reconstr Surg 2014;133:905-13. [Crossref] [PubMed]
- Scaglioni MF, Arvanitakis M, Chen YC, et al. Comprehensive review of vascularized lymph node transfers for lymphedema: Outcomes and complications. Microsurgery 2018;38:222-9. [Crossref] [PubMed]
- Ozturk CN, Ozturk C, Glasgow M, et al. Free vascularized lymph node transfer for treatment of lymphedema: a systematic evidence based review. J Plast Reconstr Aesthet Surg 2016;69:1234-47. [Crossref] [PubMed]
- Viitanen TP, Mäki MT, Seppänen MP, et al. Donor-site lymphatic function after microvascular lymph node transfer. Plast Reconstr Surg 2012;130:1246-53. [Crossref] [PubMed]
- Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg 2006;243:313-5. [Crossref] [PubMed]
- Gharb BB, Rampazzo A, Di Spilimbergo SS, et al. Vascularized lymph node transfer based on the hilar perforators improves the outcome in upper limb lymphedema. Ann Plast Surg 2011;67:589-93. [Crossref] [PubMed]
- Pons G, Masia J, Loschi P, et al. A case of donor-site lymphoedema after lymph node-superficial circumflex iliac artery perforator flap transfer. J Plast Reconstr Aesthet Surg 2014;67:119-23. [Crossref] [PubMed]
- Vignes S, Blanchard M, Yannoutsos A, et al. Complications of autologous lymph-node transplantation for limb lymphoedema. Eur J Vasc Endovasc Surg 2013;45:516-20. [Crossref] [PubMed]
- Cheng MH, Huang JJ, Nguyen DH, et al. A novel approach to the treatment of lower extremity lymphedema by transferring a vascularized submental lymph node flap to the ankle. Gynecol Oncol 2012;126:93-8. Erratum in: Gynecol Oncol 2014;135:408. [Crossref] [PubMed]
- Becker C. Microsurgical procedures: vascularized lymph node transfer from the submental region. In: Cheng MH, Chang DW, Patel KM. editors. Principles and practice of lymphedema surgery. 1st ed. Philadelphia: Elsevier Inc.; 2016:138-47.
- Poccia I, Lin CY, Cheng MH. Platysma-sparing vascularized submental lymph node flap transfer for extremity lymphedema. J Surg Oncol 2017;115:48-53. [Crossref] [PubMed]
- Sapountzis S, Singhal D, Rashid A, et al. Lymph node flap based on the right transverse cervical artery as a donor site for lymph node transfer. Ann Plast Surg 2014;73:398-401. [Crossref] [PubMed]
- Akita S, Mitsukawa N, Kuriyama M, et al. Comparison of vascularized supraclavicular lymph node transfer and lymphaticovenular anastomosis for advanced stage lower extremity lymphedema. Ann Plast Surg 2015;74:573-9. [Crossref] [PubMed]
- Barreiro GC, Baptista RR, Kasai KE, et al. Lymph fasciocutaneous lateral thoracic artery flap: anatomical study and clinical use. J Reconstr Microsurg 2014;30:389-96. [Crossref] [PubMed]
- Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015;135:277-85. [Crossref] [PubMed]
- Ciudad P, Maruccia M, Socas J, et al. The laparoscopic right gastroepiploic lymph node flap transfer for upper and lower limb lymphedema: technique and outcomes. Microsurgery 2017;37:197-205. [Crossref] [PubMed]
- Nguyen AT, Suami H. Laparoscopic free omental lymphatic flap for the treatment of lymphedema. Plast Reconstr Surg 2015;136:114-8. [Crossref] [PubMed]
- Schaverien MV, Hofstetter WL, Selber JC. Vascularized jejunal mesenteric lymph node transfer for lymphedema: a novel approach. Plast Reconstr Surg 2018;141:468e-9e. [Crossref] [PubMed]
- Lee M, McClure E, Reinertsen E, et al. Lymphedema of the upper extremity following supraclavicular lymph node harvest. Plast Reconstr Surg 2015;135:1079e-82e. [Crossref] [PubMed]
- Ciudad P, Manrique OJ, Date S, et al. A head-to-head comparison among donor site morbidity after vascularized lymph node transfer: pearls and pitfalls of a 6-year single center experience. J Surg Oncol 2017;115:37-42. [Crossref] [PubMed]
- Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg 2000;16:437-42. [Crossref] [PubMed]
- Nagase T, Gonda K, Inoue K, et al. Treatment of lymphedema with lymphaticovenular anastomoses. Int J Clin Oncol 2005;10:304-10. [Crossref] [PubMed]
- Chen WF, Yamamoto T, Fisher M, et al. The “octopus” lymphaticovenular anastomosis: evolving beyond the standard supermicrosurgical technique. J Reconstr Microsurg 2015;31:450-7. [Crossref] [PubMed]
- Yamamoto T, Kikuchi K, Yoshimatsu H, et al. Ladder-shaped lymphaticovenular anastomosis using multiple side-to-side lymphatic anastomoses for a leg lymphedema patient. Microsurgery 2014;34:404-8. [Crossref] [PubMed]
- Koshima I, Narushima M, Mihara M, et al. Lymphadiposal flaps and lymphaticovenular anastomoses for severe leg edema: functional reconstruction for lymph drainage system. J Reconstr Microsurg 2016;32:50-5. [PubMed]
- Hong JP, Choi DH, Suh H, et al. A new plane of elevation: the superficial fascial plane for perforator flap elevation. J Reconstr Microsurg 2014;30:491-6. [Crossref] [PubMed]
- Kim JH, Kim KN, Yoon CS. Reconstruction of moderate-sized distal limb defects using a superthin superficial circumflex iliac artery perforator flap. J Reconstr Microsurg 2015;31:631-5. [Crossref] [PubMed]
- Hong JP, Sun SH, Ben-Nakhi M. Modified superficial circumflex iliac artery perforator flap and supermicrosurgery technique for lower extremity reconstruction: A new approach for moderate-sized defects. Ann Plast Surg 2013;71:380-3. [Crossref] [PubMed]
- Goh TLH, Park SW, Cho JY, et al. The search for the ideal thin skin flap: Superficial circumflex iliac artery perforator flap-A review of 210 cases. Plast Reconstr Surg 2015;135:592-601. [Crossref] [PubMed]
- Feng S, Xi W, Zhang Z, et al. A reappraisal of the surgical planning of the superficial circumflex iliac artery perforator flap. J Plast Reconstr Aesthet Surg 2017;70:469-77. [Crossref] [PubMed]
- Lee KT, Park BY, Kim EJ, et al. Superthin SCIP flap for reconstruction of subungual melanoma: aesthetic functional surgery. Plast Reconstr Surg 2017;140:1278-89. [Crossref] [PubMed]
- Schacht V, Luedemann W, Abels C, et al. Anatomy of the subcutaneous lymph vascular network of the human leg in relation to the great saphenous vein. Anat Rec (Hoboken) 2009;292:87-93. [Crossref] [PubMed]
- Cho MJ, Kwon JG, Pak CJ, et al. The role of duplex ultrasound in microsurgical reconstruction: review and technical considerations. J Reconstr Microsurg 2020;36:514-21. [Crossref] [PubMed]
- Kehrer A, Sachanadani NS, da Silva NPB, et al. Step-by-step guide to ultrasound-based design of alt flaps by the microsurgeon - Basic and advanced applications and device settings. J Plast Reconstr Aesthet Surg 2020;73:1081-90. [Crossref] [PubMed]
- Visconti G, Bianchi A, Hayashi A, et al. Thin and superthin perforator flap elevation based on preoperative planning with ultrahigh-frequency ultrasound. Arch Plast Surg 2020;47:365-70. [Crossref] [PubMed]
- Kim SY, Lee YJ, Mun GH. Anatomical understanding of target subcutaneous tissue layer for thinning procedures in thoracodorsal artery perforator, superficial circumflex iliac artery perforator, and anterolateral thigh perforator flaps. Plast Reconstr Surg 2018;142:521-34. [Crossref] [PubMed]
- Jain L, Kumta SM, Purohit SK, et al. Thoracodorsal artery perforator flap: Indeed a versatile flap. Indian J Plast Surg 2015;48:153-8. [Crossref] [PubMed]
- Kim KN, Hong JP, Park CR, et al. Modification of the elevation plane and defatting technique to create a thin thoracodorsal artery perforator flap. J Reconstr Microsurg 2016;32:142-6. [Crossref] [PubMed]
- Altiparmak M, Cha HG, Hong JP, et al. Superficial circumflex iliac artery perforator flap as a workhorse flap: systematic review and meta-analysis. J Reconstr Microsurg 2020;36:600-5. [Crossref] [PubMed]
- Tang JB, Landín L, Cavadas PC, et al. Unique techniques or approaches in microvascular and microlymphatic surgery. Clin Plast Surg 2017;44:403-14. [Crossref] [PubMed]
- Kung TA, Champaneria MC, Maki JH, et al. Current concepts in the surgical management of lymphedema. Plast Reconstr Surg 2017;139:1003e-13e. [Crossref] [PubMed]
- Masià J, Pons G, Rodríguez-Bauzà E. Barcelona lymphedema algorithm for surgical treatment in breast cancer-related lymphedema. J Reconstr Microsurg 2016;32:329-35. [Crossref] [PubMed]
- Ito R, Suami H. Overview of lymph node transfer for lymphedema treatment. Plast Reconstr Surg 2014;134:548-56. [Crossref] [PubMed]
- Yamamoto T, Yamamoto N, Kageyama T, et al. Technical pearls in lymphatic supermicrosurgery. Glob Health Med 2020;2:29-32. [Crossref] [PubMed]
- Cha HG, Oh TM, Cho MJ, et al. Changing the paradigm: lymphovenous anastomosis in advanced stage lower extremity lymphedema. Plast Reconstr Surg 2021;147:199-207. [Crossref] [PubMed]
- Bianchi A, Visconti G, Hayashi A, et al. Ultra-High frequency ultrasound imaging of lymphatic channels correlates with their histological features: a step forward in lymphatic surgery. J Plast Reconstr Aesthet Surg 2020;73:1622-9. [Crossref] [PubMed]
- Schaverien MV, Munnoch DA, Brorson H. Liposuction treatment of lymphedema. Semin Plast Surg 2018;32:42-7. [Crossref] [PubMed]
- Granzow JW. Lymphedema surgery: the current state of the art. Clin Exp Metastasis 2018;35:553-8. [Crossref] [PubMed]
- Forte AJ, Huayllani MT, Boczar D, et al. Lipoaspiration for the treatment of lower limb lymphedema: a comprehensive systematic review. Cureus 2019;11:e5913. [Crossref] [PubMed]
- Damstra RJ, Voesten HGJM, Klinkert P, et al. Circumferential suction-assisted lipectomy for lymphoedema after surgery for breast cancer. Br J Surg 2009;96:859-64. [Crossref] [PubMed]
- Greene AK, Voss SD, Maclellan RA. Liposuction for swelling in patients with lymphedema. N Engl J Med 2017;377:1788-9. [Crossref] [PubMed]
- Boyages J, Kastanias K, Koelmeyer LA, et al. Liposuction for advanced lymphedema: a multidisciplinary approach for complete reduction of arm and leg swelling. Ann Surg Oncol 2015;22:S1263-70. [Crossref] [PubMed]
- Granzow JW, Soderberg JM, Dauphine C. A novel two-stage surgical approach to treat chronic lymphedema. Breast J 2014;20:420-2. [Crossref] [PubMed]
- Chen WF, Zeng WF, Hawkes PJ, et al. Lymphedema liposuction with immediate limb contouring. Plast Reconstr Surg Glob Open 2019;7:e2513. [Crossref] [PubMed]
Cite this article as: Orfahli LM, Fahradyan V, Chen WF. Vascularized lymph vessel transplant (VLVT): our experience and lymphedema treatment algorithm. Ann Breast Surg 2022;6:8.

