
Tranexamic acid use in breast surgery: a systematic review and meta-analysis
Introduction
Tranexamic acid is a synthetic amino acid that blocks plasminogen being converted to the enzyme plasmin. Plasmin works by breaking down fibrin already formed in blood clots resulting in fibrinolysis and therefore clot lysis. The use of tranexamic acid (TXA) in breast surgical procedures has been controversial, however recently there has been increased interest in the subject. This interest has been curbed by the theoretical risk of thromboembolic events particularly for cancer patients and breast reconstruction involving micro-vascular anastomoses. Mastectomy with or without axillary surgery can infrequently be complicated by haematoma and seroma formation (1,2), haematoma is also a risk for aesthetic breast operation (3).
TXA has been extensively evaluated in several fields of medicine including trauma, orthopaedics, gynaecology and cardiothoracics (4-7) and has been shown to reduce bleeding and the need for blood transfusion when administered intravenously or topically (8). Smaller studies have also demonstrated a reduction in seroma formation using TXA (9,10).
The aim of this review was to determine: (I) does tranexamic acid increase thromboembolic events in aesthetic, oncological or reconstructive breast surgical procedures and (II) does TXA reduce haematoma and seroma formation in breast surgical procedures. We present the following article in accordance to the PRISMA reporting checklist (11) (available at http://dx.doi.org/10.21037/abs-20-126).
Methods
Prior to commencing the review, a protocol was established between all authors and uploaded to PROSPERO online, study ID number 180806.
Eligibility
All randomized control studies, cohort studies and controlled before-after studies were included for assessment including prospective and retrospective studies. Review articles, expert opinion, case series, conference abstracts, posters, commentaries and non-English language articles were excluded. Studies were included if they performed breast surgery and compared tranexamic acid use to a control group. Studies were not excluded based on publication date.
Patient selection
Inclusion criteria were all types of breast surgical procedures including mastectomy with or without axillary surgery, wide local excision with or without axillary surgery, breast reconstruction either immediate or delayed, mastopexy, breast augmentation or mammoplasty procedures. Exclusion criteria included patients with known thromboembolic disease, currently taking anticoagulant medications with known coagulation disorders and pregnant patients.
Patient interventions
Patients receiving TXA as part of their surgical procedure versus either a placebo group or standard care. Administration of tranexamic acid could be intravenous, oral, topical or a combination of these routes.
Information sources
Data was extracted by two independent reviewers from online libraries, including Embase, Medline, the Cochrane Central Register of Controlled Trials, Mednar and google scholar. Study period included from database inception to 1 May 2020. Identified full text articles were examined for additional references. Results of literature search were stored on endnote online.
Search strategy
The previously mentioned libraries were reviewed using the search terms listed below and combined using Boolean operators AND and OR. Search terms used for Medline are listed in Appendix 1.
Study selection
Abstracts identified by the literature search were independently analyzed by two separate reviewers (AW and PM) looking for relevant articles for full text analysis. The above eligibility criteria were followed. Disagreements were resolved using a third party (BD). Studies were included if they met the following criteria: (I) studies involved comparison of the efficacy and safety of TXA usage in breast surgery and (II) studies included at least one of the outcome measures. All identified study authors were contacted for additional unpublished information.
Data extraction and quality assessment
Extracted data was stored on excel. Two independent reviewers assessed articles for study design, type of surgical procedure, number of patients, age, patient demographics, exclusion criteria, randomization, blinding, types of controls, outcome data including haematoma rates, seroma rates, drain volumes and thromboembolic events.
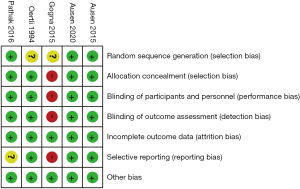
Randomized controlled study quality was assessed using the Cochrane risk of bias tool (12). This tool examines 7 areas for bias including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete data, selective reporting and other bias. Each area is assigned a judgment score of low risk, unclear risk or high risk of bias. Studies including multiple areas of unclear risk of bias or an area of high risk were considered high risk of bias and excluded from the meta-analysis.
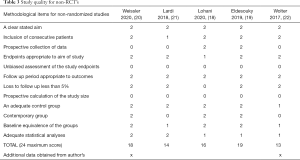
For non-randomized studies, quality was assessed using the methodological index for non-randomized studies (MINORS) (13) which is designed to assess the quality of non-randomized interventional studies. Studies were assessed independently by two reviews using the 12-question model. Each question can be assigned 0–2 points for a maximum score of 24. Zero points for unreported, one point for reported but inadequate, two points for reported and adequate. A score of ≥18 was considered high quality, 14–17 as moderate quality and ≤13 low quality. Non-RCT studies were excluded from the meta-analysis.
Statistical analysis
The meta-analysis was conducted using Revman Review Manager 5.3 software. For dichotomous data, the odds ratio (OR) with 95% confidence intervals (CI) were used. For continuous outcomes, a weighted mean difference (MD) and 95% CI were used. If the above data points were missing, they were calculated from available data using Revman software. Heterogeneity of the studies was assessed using Cochran’s Q test and a significance value (P) <0.10 was used. The I2 test looking for observed total variation across studies due to real heterogeneity and not chance was also used. A value of 0% indicates no observed heterogeneity, whereas a larger value shows increased heterogeneity. The random effects model was used for all calculations due to variability in study design. In the random effect model, the true effect is assumed to vary between studies and the summary effect is the weighted average of the effects in the different studies. The assessment of publication bias and meta-regression was not conducted because at least 10 studies are usually required to perform these tests and the most frequent reported outcome, haematoma rate, was only reported in 4 included studies.
Ethical approval
All analyses were based on previously published data, with no new patient contact and therefore ethical approval was not requested.
Results
Search results
A total of 2,038 abstracts were identified. This number was reduced to 1,705 after duplicates were removed: Medline [734], Embase [208], Cochrane [189], Mednar [572] and google [2]. After abstract review, we excluded 1667 as these abstracts were deemed irrelevant as they either related to breast surgery but not tranexamic acid, tranexamic acid but not breast surgery or neither tranexamic acid nor breast surgery. This left 38 papers for full text screening. One review article and two letters to the editor were excluded. Seventeen duplicate papers and five research projects either awaiting commencement or in progress but not completed, were excluded. Two abstracts were then excluded from the study and one commentary, leaving 9 articles. After liaising with one of the abstract authors his paper is since published and has been included in the review for a total of 10 papers. A flowchart of this is demonstrated in Figure 1. Five RCT’s (10,14-17) and five non-RCT’s including 2 prospective cohort studies (18,19), 2 retrospective cohort studies (20,21) and one controlled before-after study (22) were identified. Seven articles were located from Embase and Medline (10,14,15,18,20,21), 3 articles from grey databases (16,17,19). The characteristics of all included studies are shown in Tables 1 and 2.
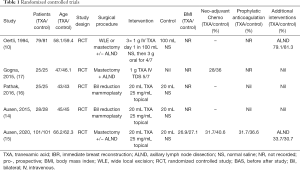
Full table
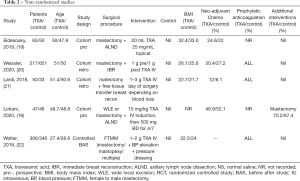
Full table
Study quality
The quality of RCT’s are shown in Figure 2. Four studies were rated as low risk of bias (10,14-16) and one as a high risk of bias (17). In two studies the process of randomization was explicitly detailed (14,15), whereas in the other three randomization was stated but not fully explained (10,16,17). Blinding of patients and clinicians was universal, except in one study (17), however in three studies outcome assessors blinding could not be determined (10,16,17). In all studies due to short follow up periods dropout rates were minimal with good compliance and intention to treat analyses were performed. No co-interventions were introduced.
The quality of non-randomized studies (18-22) (non-RCT) are shown in Table 3. One study (19) had a MINORS score of ≥18 and was deemed high quality, three studies moderate quality (18,20,21) and one study was low quality (22) with a score of ≤13 or less. Two studies collected data prospectively, while the others were retrospective. None of the non-RCT’s used blinding of study outcomes and none of the studies made a prospective calculation of study size. Most had adequate control groups, however one study (22) did incorporate variations in surgical technique and the use of pressure dressings post operatively, as well as TXA to the intervention group. Two studies used historical data as a control group (21,22), rather than contemporary groups. Three studies (20-22) had significant differences between the groups at baseline which may have affected outcomes. Most studies had adequate follow up and retention, except one (19) which had a 5% loss to follow up for the main outcome.
Full table
Studies included in meta-analysis
Of the four RCT’s (10,14-16) two studies included WLE or mastectomy patients with or without axillary surgery (10,15). This included 180 breasts in the TXA groups and 182 in the control groups. Two other studies (14,16) looked at bilateral reduction mammoplasties and compared one side to the other, they included 53 patients and 106 breasts.
Three studies (14-16) examined topical application of TXA to the wound bed, including 154 breasts in the TXA groups and 154 breasts in the control groups, the other studies regime (10) involved IV TXA initially followed by oral TXA for four days including 79 patients in the TXA groups and 81 in the control groups.
Excluded studies from meta-analysis
One study (17) was excluded from the analysis due to the high risk of bias. No placebo was not given, unlike the other studies, and therefore blinding for staff and patients was unlikely.
Effects of interventions
Figure 3 is a summary of findings for the main outcomes.
Thromboembolic events
Due to a low number of events a meta-analysis could not be performed for this outcome. Excluding the 2 papers on patients with bilateral mammoplasties used as their own controls. In eight studies in the review only one thrombotic event was recorded in the control group for a venous anastomosis in a free flap patient (21). Zero patients out of 950 patients in the TXA group and 1 patient out of 1,333 patients in the control group (P=0.35). TXA did not increase the risk of thromboembolic events.
Haematoma rates
Three studies (10,14,15) compared haematoma rates. In the TXA group 6/208 breasts versus 13/210 breasts in the control group. Overall the studies had moderate heterogeneity (P=0.15, I2=48%), Figure 4. The pooled results suggest no effect on haematoma rates after TXA (OR =0.42, 95% CI: 0.19 to 0.76, P=0.30).
Drain fluid and seroma
Drain volume over 1st 24 hour’s
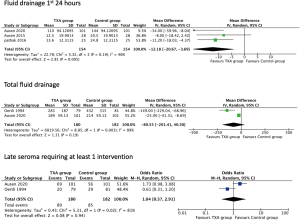
Three studies (14-16) reviewed seroma fluid drainage over the first 24 hours after surgery. Both arms included 154 breasts. Mean drain volume was 102 mL for the control group and 90 mL for the TXA group. Heterogeneity between studies was moderate (P=0.19, I2=40%, Figure 5). The overall result was significant favouring TXA group (MD =−12 mL, 95% CI: −20.7 to −3.7, P=0.005).
Total drain volume
Two studies (10,15) looked at total drainage volume prior to removal. This included 180 patients in the TXA group with mean drainage of 230 mL versus the control group with 182 patients and 310 mL. Heterogeneity was high (P=0.003, I2=89%, Figure 5). The overall result suggests no difference with TXA (MD =−80.5, 95% CI: −201.4 to 40.3, P=0.19).
Late seroma requiring intervention
Two studies (10,15) looked at late seroma rates, defined as requiring aspiration or further intervention at least once. In the TXA group 89/180 required an intervention versus 85/182 in the control arm. Heterogeneity was noted to be high (P=0.02, I2=81%, Figure 5). The overall results suggest no significant effect from TXA, (OR =1.04, 95% CI: 0.37–2.91, P=0.94).
Long term outcomes
None of the included studies looked at long term outcomes.
Discussion
Summary of main results
For mastectomy patients with or without axillary surgery combined with mammoplasty no effect on haematoma formation, overall drainage or late seroma formation was seen. A reduction in drainage fluid at 24 hours after surgery was noted.
Overall completeness and applicability of evidence
The results of this review need to bear in mind that they are derived from a small number of individually underpowered studies with few patients. Small studies may be powered enough to detect differences in drain volumes, but underpowered to detect differences in haematoma rates, a complication seen in <5% of patients. Many of the studies in this field are non-randomized studies that may over-estimate treatment effects, with few RCT’s remaining. Unfortunately, no reconstructive papers were included in the final meta-analysis so the conclusions of this study cannot be applied to immediate or delayed breast reconstructive procedures or aesthetic surgical procedures including mastopexy or augmentation.
Agreement and disagreement with other studies and reviews
TXA use has been extensively reviewed in the setting of major surgery and trauma where it has been shown to reduce bleeding, the need for blood transfusion and death (7,23) and is now recommended as an early intervention. Orthopaedic studies looking at the safety of TXA in hip and knee joint replacement surgery found no increased risk of thromboembolic events compared to controls (24,25). In contrast, the recent HALT-IT study (26) found an increase in deep vein thrombosis and pulmonary embolism with TXA use in acute gastrointestinal bleeding amongst 12,009 patients, with no benefits to mortality, bring in to question the safety of TXA. An increased risk of venous thromboembolism in 21,931 trauma patients was demonstrated in a retrospective propensity matched study (27), again with no associated gain in survival. Potentially this increased in thromboembolic risk is only seen in large studies due to the rarity of the event. Less is known about the benefits of TXA in surgery where major haemorrhage is less common. In 2015 the journal of military medicine published a retrospective cohort study (28) which found no increased risk of thromboembolic events, including flap thrombosis, for patients undergoing extremity reconstructions in combat care treated with TXA. In plastic surgery TXA was found to decrease blood loss in liposuction (29) and reduce oedema and ecchymosis in septorhinoplasty patients (30).
Limitations
The major limitations for this review include four main factors: (I) the lack of randomized controlled trials, (II) the types of surgical interventions, (III) the routes of TXA administration and (IV) outcome measures.
Lack of randomized trials
Many of the questions this review hope to answer could not be due to a lack in randomized trials. Only patients who underwent wide local excision or mastectomy with or without axillary surgery (10,15), as well as mammoplasty patients (14,16) were included in the meta-analysis. Two non-RCT papers examining breast reconstruction were excluded, one on implant-based reconstruction (20) and another on autologous reconstruction (21). The implant reconstruction paper did demonstrate a reduction in haematoma rates for the TXA group controlled for age, hypertension and implant position (pre-pectoral versus subpectoral) and mastectomy type (nipple versus skin sparing mastectomy) in 868 patients (P=0.018). They also noted a non-significant reduction in seroma formation. The free-flap autologous reconstruction paper included 83 patients noting no effect on haematoma formation (P=0.332), but a significant reduction in blood loss, 70 mL on average (P<0.001).
Variation in surgical interventions
Even in the small number of papers included in the final meta-analysis variations in surgical procedures is a major limiting factor. The difference in volumes of drained fluid from a wide local excision versus a mastectomy complicated by variations in axillary surgery is self-evident. Lack of benefit or benefits may be masked by grouping such procedures together, even with randomization. Bilateral mammoplasty provides an excellent control for studies, however the volume of fluid drained and the risk of late seroma are far less then compared to mastectomy.
Route of administration of TXA.
In the meta-analysis three studies (14-16) reviewed topical TXA as a single dose at the time of surgery and one study (10) three IV TXA doses in the first 24 hours followed by oral doses for a further 4 days. Bilateral mammoplasty acts as an excellent control group when topical TXA is administered but currently the effects systemically from this are not known and may affect the overall results. The best methods of administration of TXA for surgical patients is still debated and varies depending on the indications. One review (31) noted a reduction in blood loss and transfusion requirements with the majority of patients receiving a single IV dose of 15 mg/kg of TXA. The beneficial effects of topical TXA on blood loss and transfusion requirement have also been studied and confirmed by a meta-analysis of surgical patients including 71 trials and 7,539 patients (8). They noted no increase in adverse events including mortality, pulmonary embolism, myocardial infarction, deep vein thrombosis or stroke. Fatima et al. (32) reviewed topical tranexamic acid versus placebo in spinal deformity surgery and demonstrated no difference in complications between the two groups but noted a decrease in drain output for the TXA group and hospital stay. The best method of administration in breast surgery is still to be determined.
Outcome measures
Late seroma formation requiring at least one intervention is unaffected in this meta-analysis from limited studies, however from this data we do not know if the number of interventions required by the patient is affected by TXA.
Quality of evidence and Grade
This study has demonstrated that TXA may have no effect on thromboembolic risk for breast surgery patients, but the certainty of evidence is low due to numbers of patients, mixed operation types and incorporating non-RCT’s for this outcome. For mastectomy patients with or without axillary surgery and mammoplasty patients the rate of haematoma formation, after TXA, probably results in little to no difference with moderate certainty. Drain fluid output in the first 24 hours is reduced with high certainty, but the effect is small and may not be important overall to the patient. Drain production overall is lower but does not reach significance with moderate certainty and may not be important. Seroma formation requiring at least one intervention is unaffected by TXA to moderate certainty for mastectomy patients with or without axillary surgery.
Conclusions
The overall benefits and risks of TXA remain uncertain in breast surgery. Further larger scale RCT’s are required to determine the real benefit from TXA in breast surgery, what dose, the best route of administration and in which surgical procedures. Two incomplete studies were identified during the literature search on clinicialtrials.gov, one “Tranexamic acid for bleeding in breast surgery (TABBS)” (33), which had so far failed to secure funding, and the second “Seroma Reduction pOst Mastectomy (SEROMA study)” (34) which is in recruitment and may provide addition information on this subject.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-126
Peer Review File: Available at http://dx.doi.org/10.21037/abs-20-126
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-126). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nwaogu IY, Bommarito K, Olsen MA, et al. Economic impact of bleeding complications after mastectomy. J Surg Res 2015;199:77-83. [Crossref] [PubMed]
- Carless PA, Henry DA. Systematic review and meta-analysis of the use of fibrin sealant to prevent seroma formation after breast cancer surgery. Br J Surg 2006;93:810-9. [Crossref] [PubMed]
- Gupta V, Yeslev M, Winocour J, et al. Aesthetic Breast Surgery and Concomitant Procedures: Incidence and Risk Factors for Major Complications in 73,608 Cases. Aesthet Surg J 2017;37:515-27. [Crossref] [PubMed]
- Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ 2014;349:g4829. [Crossref] [PubMed]
- Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev 2018;4:CD000249 [PubMed]
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomized, double-blind, placebo-controlled trial. Lancet 2017;389:2105-16. [Crossref] [PubMed]
- CRASH-2 trial collaborators, Shakur H, Roberts I, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomized, placebo-controlled trial. Lancet 2010;376:23-32.
- Teoh WY, Tan TG, Ng KT, et al. Prophylactic Topical Tranexamic Acid Versus Placebo in Surgical Patients: A Systematic Review and Meta-Analysis. Ann Surg 2021;273:676-83. [Crossref] [PubMed]
- Albatanony A, Shahin M, Fayed A, et al. The effects of intravenous tranexamic acid on reduction of seroma after para-umbilical hernioplasty. Surg J 2019;6:2290-4.
- Oertli D, Laffer U, Haberthuer F, et al. Perioperative and postoperative tranexamic acid reduces the local wound complication rate after surgery for breast cancer. Br J Surg 1994;81:856-9. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff JThe PRISMA Group, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6:e1000097 [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PCCochrane Bias Methods Group, et al. Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Slim K, Nini E, Forestier D, et al. Methological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 2003;73:712-6. [Crossref] [PubMed]
- Ausen K, Fossmark R, Spigset O, et al. Randomized clinical trial of topical tranexamic acid after reduction mammoplasty. Br J Surg 2015;102:1348-53. [Crossref] [PubMed]
- Ausen K, Hagen AI, Ostbyhaug HS, et al. Topical moistening of mastectomy wounds with diluted tranexamic acid to reduce bleeding: randomized clinical trial. BJS Open 2020;4:216-24. [Crossref] [PubMed]
- Pathak S, Husain A, Sharma S, et al. Assessing the role of topical tranexamic acid application post-reduction mammoplasty patients. Int J Cont Med Res 2016;3:1529-31.
- Gogna S, Goyal P. Prospective randomized study on effect of tranexamic acid on wound drainage following modified radical mastectomy for cancer breast. Int J Cur Res 2015;7:16192-4.
- Lohani KR, Kumar C, Kataria K, et al. Role of tranexamic acid in axillary lymph node dissection in breast cancer patients. Breast J 2020;26:1316-20. [Crossref] [PubMed]
- Eldesouky MS, Abo Ashour HS, Shahin MA. Effect of topical application of tranexamic acid on reduction of wound drainage and seroma formation after mastectomy. Egyptain J Surg 2019;38:772-5.
- Weissler JM, Banuelos J, Jacobson SR, et al. Intravenous Tranexamic Acid in Implant-Based Breast Reconstruction Safely Reduces Hematoma without Thromboembolic Events. Plast Reconstr Surg 2020;146:238-45. [Crossref] [PubMed]
- Lardi AM, Dreier K, Junge K, et al. The use of tranexamic acid in microsurgery – is it safe? Gland Surg 2018;7:S59-63. [Crossref] [PubMed]
- Wolter A, Scholz T, Pluto N, et al. Subcutaneous mastectomy in female-to-male transsexuals: Optimizing perioperative and operative management in 8 years clinical experience. J Plast Reconstr Aesthet Surg 2018;71:344-52. [Crossref] [PubMed]
- . CRASH-3 trail collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet 2019;394:1713-23. [Crossref] [PubMed]
- Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ 2014;349:g4829. [Crossref] [PubMed]
- Alshryda S, Sukeik M, Sarda P, et al. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J 2014;96-B:1005-15. [Crossref] [PubMed]
- HALT-IT Trial Collaborators. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet 2020;395:1927-36. [Crossref] [PubMed]
- Myers SP, Kutcher ME, Rosengart MR, et al. Tranexamic acid administration is associated with an increased risk of posttraumatic venous thromboembolism. J Trauma Acute Care Surg 2019;86:20-7. [Crossref] [PubMed]
- Valerio IL, Campbell P, Sabino J, et al. TXA in combat casualty care - does it adversely affect extremity reconstruction and flap thrombosis rates? Mil Med 2015;180:24-8. [Crossref] [PubMed]
- Cansancao AL, Conde-Green A, David JA, et al. Use of tranexamic acid to reduce blood loss in liposuction. Plast Reconstr Surg 2018;141:1132-5. [Crossref] [PubMed]
- Sakallioğlu Ö, Polat C, Soylu E, et al. The efficacy of tranexamic acid and corticosteroid on edema and ecchymosis in septorhinoplasty. Ann Plast Surg 2015;74:392-6. [Crossref] [PubMed]
- Heyns M, Knight P, Steve AK, et al. A Single Preoperative Dose of Tranexamic Acid Reduces Perioperative Blood Loss: A Meta-analysis. Ann Surg 2021;273:75-81. [Crossref] [PubMed]
- Fatima N, Barra ME, Roberts RJ, et al. Advances in surgical hemostasis: a comprehensive review and meta-analysis on topical tranexamic acid in spinal deformity surgery. Neurosurg Rev 2021;44:163-75. [Crossref] [PubMed]
- Rockwell G. Tranexamic acid for bleeding in breast surgery (TABBS). ClinicalTrials.gov Identifier: NCT02615366
- Duchesnay I. Seroma Reduction pOst Mastectomy “SEROMA study”. Clinicatrials.gov Identifier: NCT03738527.
Cite this article as: Winder AA, McQuillan P, Dijkstra B. Tranexamic acid use in breast surgery: a systematic review and meta-analysis. Ann Breast Surg 2021;5:12.




