
Renal transplant recipients suffer significantly more complications but not mortality after breast cancer surgery and benefit from treatment at transplant centers
Introduction
The number of kidney transplants (KT) has increased annually over the past decade (1). Survival rate among KT recipients (KTR) has also improved in that time period, closely mirroring improvements in graft survival. There were nearly 220,000 individuals living with a renal allograft in 2017 (2). History of an organ transplant is a known risk factor for developing a malignancy (3). As the rates of KTs continue to climb, the number of KTR diagnosed with cancer is likely to grow.
Breast carcinoma is the leading cause of new cancer diagnosis in women (4). It was also identified in 4.7% of de novo cases in those with history any transplant (5). Breast carcinoma was the most frequently encountered gynecologic malignancy in KTR (6). They are also more likely to have advanced disease burden at diagnosis (7). Others observed higher mortality in renal allograft recipients diagnosed with Stage III or Stage IV breast cancer (8). However, there is limited information on the management and outcomes in KTR. Single center trials have demonstrated comparable prognoses between transplant and non-transplant cohorts (9).
It is imperative to develop a greater understanding of the outcomes of breast surgery in KT patients. Our purpose is to evaluate the influence of KT history on the short-term outcomes of mastectomy or lumpectomy in women at transplant and non-transplant centers. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/abs-20-56).
Methods
The Nationwide Inpatient Sample (NIS) is the largest database of hospital discharges in the United States. It is a product of the Healthcare Cost and Utilization Project and includes approximately 7 million annual hospitalizations. NIS enables researchers to formulate national estimates because of its 20% stratified sample. We obtained NIS data from 2005 to 2014 and classified individuals who underwent lumpectomy or mastectomy (ICD 9 code: 85.20–85.23, 85.33–85.36, and 85.41–85.48). Within this subset, we selected for those with history of KT (ICD9: V42.0).
Persons with a history of another organ transplant (ICD9: V42.1, V42.2, V42.6, V42.7, V42.8, V42.81, V42.82, V42.83, V42.84, V42.89, V42.9) were excluded. Individuals with complications from prior organ transplants were also excluded (ICD9 codes: 996.80, 996.82, 996.83, 996.84, 996.85, 996.86, 996.87, 996.88, 996.89). Other exclusion criteria were benign tumor, age younger than 18 years, and male gender. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
After applying exclusion and inclusion criteria, hospital and patient-level characteristics between KT and non-KT recipients were compared with t-test, Mann-Whitney test, and chi square test. In order to adjust for comorbidities, Elixhauser Comorbidity Index (ECI) scores were calculated from NIS data. The outcomes of interest were mortality, complications, total hospital charge and length of stay. Logistic regression models were created for mortality and complications as binary outcomes. Linear regressions were used to build models for length of stay and charges as numeric outcomes to find the role of KT as a risk factor. In addition to the multivariate logistic regression, we adjusted and normalized total charges and length of stay to further minimize bias. Total charges were adjusted based on consumer price index (CPI) 2020. We observed that both total charges and length of stay were positively skewed. We normalized these two variables by creating log transformation. This was the best method to normalize the data without changing the direction of skew. Since there was no mortality in the KTR cohort, only three of the outcomes were assessed in a multivariate fashion.
Multivariate logistic regressions were performed to compare short-term breast surgery outcomes sorted by hospital designation. Specifically, we analyzed the results for sub-cohorts of KTR treated at transplant centers (TCs), teaching organizations, and all hospitals. We identified TC as locations with at least one transplant performed during the timeframe of the study. Teaching institutions were defined by the presence of an Accreditation Council for Graduate Medical Education (ACGME) approved residency program, membership in the Council of Teaching Hospitals (COTH), or a resident to bed ratio of 0.25 or higher. Finally, we considered outcomes for allograft recipients undergoing immediate breast reconstruction following lumpectomy or mastectomy at TC.
The NIS database has the advantage of collecting both patient and hospital characteristics. Patient and hospital characteristics had some statistically significant and non-significant values. To reduce any bias, we entered all the patient and hospital characteristics in the multivariate logistic and linear regression models along with the surgical procedures because they were either statistically significant or clinically relevant. All results were calculated after applying the sampling weight built in NIS.
Results
Demographics
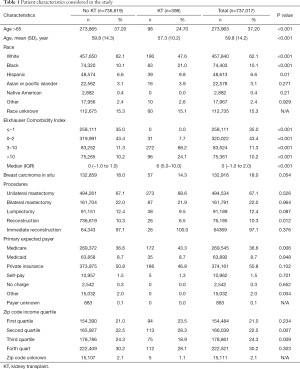
The sample population consisted of 737,017 individuals who underwent lumpectomy, unilateral mastectomy, or bilateral mastectomy between 2005 and 2014. In this group, 398 had a prior KT after application of inclusion and exclusion criteria. Demographic characteristics are outlined in Table 1. An assessment demonstrated that KTR obtaining treatment were significantly younger (57.3 vs. 59.8, P<0.001) and had fewer individuals older than 65 (24.70% vs. 37.2, P<0.001). The overall breast operation population was predominantly made up of white females (62.1%), however they constituted a smaller portion of the KT cluster (47.6%, P<0.001). In comparison, a greater percentage of African-American (21.0% vs. 10.1%, P<0.001) and Hispanic (9.8% vs. 6.6%, P=0.01) women undergoing surgery were transplant recipients than otherwise. The overall rate of carcinoma in situ was 18.0%. There was no significant difference based on KT and non-KT (14.3% vs. 18.0%, P=0.054).
Full table
ECI
Participants were classified into ECI categories <−1, 0–2, 3–10, and >10. Our analysis demonstrated that KTR had a higher median comorbidity score (6) compared to the non-transplant division (0). Significantly fewer with an allograft were in the <−1 (P<0.001), and 0–2 (P<0.001) groups. Furthermore, meaningfully more recipients were in the 3–10 (P<0.001), and >10 (24.1% vs. 10.2%, P<0.001) comorbidity index classes.
Procedures
The most common surgical treatment was unilateral mastectomy (67.1%). Breast reconstruction was performed in 10.3% of cases. In those instances, 97.1% of reconstructions were immediate. Significantly fewer transplant recipients underwent reconstruction (6.5% vs. 10.3%, P=0.012). There was no variation between immediate and delayed reconstruction based on KT history, but all recipients underwent immediate reconstruction.
Insurance
A majority of the entire operative population was privately insured (50.8%). However, Medicare was the primary payer for significantly more KTR (43.3% vs. 36.6%, P=0.006). There was no difference in median household incomes across quartiles 1 and 4 of the transplant and comparison group. There were significant variances in quartiles 2 and 3, with most KTR located in quartile 2 (28.3% vs. 22.5%, P=0.007).
Hospital characteristics
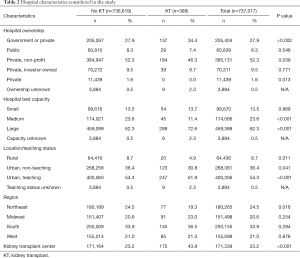
Hospital characteristics are compiled in Table 2. We noted that significantly more women with a KT received operative management for breast cancer at large hospitals by bed number (P<0.001). Comparatively, those without renal replacement surgery visited medium sized hospitals more frequently (P<0.001). More KTR sought treatment at TCs (P<0.001).
Full table
Private non-profit hospitals were the site for most breast surgeries. KTR preferentially utilized government owned centers compared to the cohort (34.4% vs. 27.9%, P<0.002). Transplant patients obtained care at urban, teaching hospitals (61.9% vs. 54.3%, P<0.001) and less likely to visit rural (P=0.011), or non-teach urban institutions (P=0.041). Fewest kidney recipients underwent breast cancer surgeries in the Northeast (P=0.016).
Outcomes
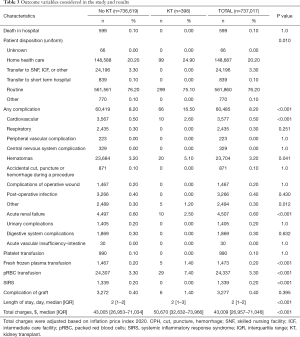
Table 3 features the short-term results of univariate analysis for breast surgeries. The renal transplant population had significantly different outcomes. There was no mortality in the KTR, but complication rates were nearly double (16.5% vs. 8.2%, P<0.001). Specifically, the most frequently encountered complications were cardiovascular (P<0.001), acute renal failure (P<0.001), hematomas (P=0.041), and other (P=0.012). Furthermore, allograft recipients required more fresh frozen plasma (P<0.001), and packed red blood cell (pRBC) transfusions (P<0.001) after surgery. KTR had longer length of stay after their procedures (P<0.001) along with higher total expenditures (P<0.001). Median charge was nearly $8,000 more for treatment. Despite this, no one with an allograft met SIRS criteria compared to 0.2% for the cohort (P<0.001).
Full table
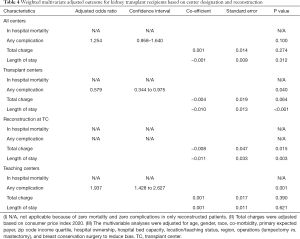
Weighted multivariate adjusted outcomes for all KTR based on transplant, and teaching designations can be found in Table 4. Weighted analyses highlight meaningful differences compared to other hospitals. TC illustrated significantly lower rates of suffering any complication after operations (P=0.040) and shorter length of stay (P<0.001). Teaching institutions produced a higher adjusted odds ratio (aOR) for any complication (1.937, P=0.001). Those KTR with immediate breast reconstruction surgery benefitted from a lower total charge (P=0.015) and shorter LOS (P=0.003) at transplant hospitals.
Full table
Discussion
History of solid organ transplant (SOT) is linked to an increased risk of developing a malignancy (3). Our aim was to investigate short term post-operative outcomes in women with KT undergoing surgery for breast cancer at transplant and non-transplant centers. Previous publications have examined surgical results in kidney recipients. After undergoing appendectomies and cholecystectomies, they had increased expenses and length of stay (10,11) KTR also sustained increased expenses, length of stay, overall complication, and mortality rate after colorectal resection (12).
A demographic analysis outlined statistically significant variations. KTR undergoing breast operations were generally younger. The mean age was 57.3 years as opposed to 59.8 in the cohort. The percentage older than 65 years was also meaningfully lower. This is not unexpected. Organ transplant and immunosuppressive treatments are well established risk factors for malignancies (3). These are likely contributing factors in the development of breast cancer at an earlier age.
Considerably greater proportions of African-American and Hispanic women with KT required surgical intervention for breast carcinoma. These findings are particularly interesting due to the demographic prevalence of breast cancer and KT. Since 1988, more than twice as many White females (n=100,378) as African American females (n=46,196) underwent KT. An even smaller number of Hispanic women (n=26,102) received a renal allograft over the same time period (1). The age-adjusted rate of breast cancer per 100,000 has been very similar between Whites (126.1) and African Americans (124.0) whereas the Hispanic rates were far lower (93.9) (13). Although our analysis was limited by size, the appreciably higher number of African American and Hispanic females in the sample was curious and of note nonetheless. Researchers have encountered greater tacrolimus clearance in African-Americans, compared to Non-Hispanic Whites, which require higher doses to reach therapeutic levels (14). Others concluded that Hispanic KTR do not suffer similar effects and in fact require less immunosuppressive therapy (15). The variability in drug regiment and dosing could explain increased rates of breast malignancy in American-American and Hispanic women compared to their non-transplanted cohort.
The ECI uses 31 unique diagnostic categories to predict charges, length of stay, and in-patient mortality (16). Each condition is assigned a point value ranging from −7 to +12, corresponding to the strength of association with death prior to discharge. Next, an algorithm is used to generate a score between −19 and +89 (17). In general, a higher ECI score points to an increased likelihood of mortality and provides a good framework to measure disease burden and resource utilization. Our investigation demonstrated that significantly more KTR with breast cancer had ECI between 3–10 (68.2%) and over 10 (24.1%), which was drastically higher than the 11.3% and 10.2%, in the higher Elixhauser categories respectively, for those without prior transplants.
This paints the KTR cohort as a generally sicker population with numerous risk factors requiring more hospital resources and placing them at increased risk of in-hospital mortality. This is not entirely unexpected as several conditions that heavily influence ECI are common in transplant recipients. Comorbidities such as hypertension, diabetes mellitus (DM), DM with complication, and renal failure can be precursors to KT. Whereas coagulopathies, fluid and electrolyte abnormalities, and anemias are seen in KTR and they are important considerations in the ECI scoring.
A substantially larger segment of the KTR utilized Medicare as the primary payer for their breast cancer procedures despite a minority of patients being over 65. There were no marked differences in coverage by Medicaid, private insurance, or self-pay. It is important to note that those under 65 with end stage renal disease and an allograft are covered by Medicare for 36 months after their transplant. The variations in insurance coverage paired with the younger age of KTR point to additional factors that may be involved. We could not track duration of time between KT and breast carcinoma treatment.
A review of short-term breast cancer surgery outcomes across the study populations featured some distinct outcomes. We discovered that KTR had longer post-operative hospital stays. The median length of hospitalization was the same at 2 days. However, the range was larger in the KTR group with 1–3 days compared to 1–2 days in the control.
We identified that total hospital charges were higher in the transplant cohort. Median expenditure was nearly $8,000 higher. The increased length of stay and cost match the outcomes predicted by the ECI given the majority were in the high risk 3–10 and >10 comorbidity score clusters.
Next, KTRs were more likely to require pRBC and fresh frozen plasma transfusions during their breast surgery recovery. Prior research has indicated that anemia is a common and under-treated diagnosis following kidney transplantation (18). Although prevalence of anemia decreases following KT, it can persist for years in up to one third of cases (19).
Finally, transplant recipients experienced nearly double the rate of post-operative complications at 16.5% compared to 8.2%. Our analysis specifically tracked any short term in-hospital adverse events. This is consistent with previous publications that have categorized the influence of prior renal transplant on future surgical outcomes. In cases of cholecystectomy, appendectomy, and colorectal resection the reports confirmed that KTR experienced more in-hospital complications compared to others (10-12). Similar to our results, all three of these projects emphasized rates of any short-term ailments.
Detailed evaluations of immediate adverse events pointed to markedly increased rates of problems with hematomas, acute renal failure, and the cardiovascular system. Hematomas are likely a product of underlying anemia and coagulopathies associated with KT (18). Intuitively, one can point to a decreased baseline renal function in KTR, due to only one functioning kidney and nephrotoxic immunosuppressants as the cause behind cardiovascular and nephrology difficulties. Pre-existing comorbidities in recipients with high ECI scores are predictably a contributing factor. Challenges in managing post-operative fluid status paired with compromised filtration is likely the key in developing either of these complaints.
Others have focused on breast procedure outcomes in SOT recipients. They did not find an increased risk of complications (20). However, there were significant differences in the population and procedures tracked in these projects. Zellner et al. examined maladies following plastic surgery in SOT patients, but the sample only included 14 breast procedures across all organ transplants not limited to KT, and the majority of the cases were minor reconstruction revisions (n=6) and reduction mammoplasty (n=5) (20). In contrast, we specifically considered mastectomies and lumpectomies for breast cancer. Ongoing malignancies also carry unique risks that would not be evident in plastic surgery cases. For instance, postoperative venous thromboembolism is a known consequence of cancer, even in those established on oral anticoagulation and would necessitate additional monitoring (21,22). Nonetheless, Zellner et al. revealed a complication rate of 14% in breast procedures, which is similar to our 16.5% rate (20).
We established that KTR were less likely to undergo reconstruction after mastectomy. There was no significance in rates of immediate reconstruction or variations in outcomes based on reconstruction status. Other researchers focusing on breast reconstruction after SOT also did not discern a difference in adverse events but observed 8 instances of minor side effects such as infected seromas and cellulitis. Their series only included 7 KTR in the total 17, and the research only focused on reconstructions and not operations to reduce tumor burden (23). Both examinations of plastic surgery after organ transplant suffered from KT sample size limitations. This makes stratification and comparisons across all SOT challenging given the nuances of post-operative immunosuppression and unique risk factors associated with different organ systems. For example, DM increases the possibility of breast wound infection after operations (21). DM is a common underlying condition in renal recipients and increases the risk of complications for KTR compared to another SOT.
Features of treatment centers were evaluated and correlated with patient outcomes. More KTR sought surgical care for breast cancer at large hospitals by bed capacity compared to the cohort. The majority of the treatment facilities were urban, teaching institutions. Comparatively fewer chose urban, non-teaching or rural establishments.
Private, non-profit hospitals were the most common source of treatment for all. KTR visited government owned centers more frequently than the comparison. It is plausible that this is an artifact of the abundant Medicare beneficiaries with an allograft.
There was also a preference for TCs for breast cancer surgery amongst KTR compared to the cohort. This is consistent with the current understanding in the literature. Nationwide investigations observed that more KTR underwent colorectal resection, appendectomy, and cholecystectomy at a TC (10-12). Some published opinion-based recommendations suggest transfer of care of transplant recipients even for non-allograft operations to TC because of their familiarity with the individual and expertise in management (24,25). It can also be postulated that KTR may choose to return to their TC for continuity of care.
A broader examination of the TCs in KTR breast cancer surgery highlighted their superior performance. We identified that individuals treated at TC had lowers rates of any complications, shorter post-operative stay, and lower expenditures. Prior works by DiBrito et al. have scrutinized hospital specific complications rates for abdominal procedures and concluded there were no differences by TC status (10-12). Nevertheless, it has been well established that the complications of breast surgeries differ from those of abdominal operations (21). The inherent differences in the types of procedures likely explains this discrepancy.
Next, post-operative length of stay was shorter at TC. This was again in contrast to the evidence for KTRs at TCs after colorectal resection and appendectomy (11,12). The differences in breast and abdominal complications again likely explain these differences. Breast operations have the benefit of not interfering with the prior KT site or incision and would reasonably require less intensive post-operative oversight for any graft complications. Furthermore, it may be speculated that TCs are better equipped to monitor the nuances of renal function in the KTRs and therefore more comfortable discharging patients earlier after breast surgery.
Total expenditures after breast surgeries were marginally lower at TCs compared to non-TCs but not statistically significant (P=0.064). Again, this alters the current understanding for cost of care at TCs for procedures. DiBrito et al. identified that charges were higher after appendectomies and did not vary significantly for colorectal resection and cholecystectomies (11). It can be inferred that the lower expenditure is a function of the overall performance of TC after breast surgeries with fewer complications and a shorter length of stay.
TCs were further separated into teaching or non-teaching centers. We determined that teaching TC were associated with a higher risk of any complication. Yet they did not have any differences in LOS and total expenditure. DiBrito et al. adjusted the calculations based on teaching status but did not evaluate its impact on outcomes. A review of US hospitals discovered an association between teaching status and reduced mortality (26). These results were intriguing and require further examination to determine the underlying cause of the increased complications.
We also scrutinized the outcomes for breast reconstruction after mastectomy or lumpectomy at a TC. No mortality or complications were reported. There was an association between TCs and lower total charge for care and shorter post-operative length of stay. These findings are likely attributable to the expertise of TC in treating renal recipients.
Limitations
This investigation has a few limitations primarily stemming from the design and limited sample size of KTR undergoing surgical treatment for breast carcinomas. The project relies on accurate diagnostic and procedural coding across the country for the NIS database. Next, our sample size far lower than those used by DiBrito et al. for the abdominal surgery series (10-12). Exclusion criteria and the narrow focus on breast cancers limited the sample. However, our analysis revealed robust significance across several categories. Furthermore, a single center trial estimated the incidence of post-transplant breast cancer at 0.5% after KT (9). There were approximately 200,907 people living with a functioning renal graft in 2014 (27). Transplant data between 1988–2014 suggests that women were the beneficiaries of roughly 40% of all KT (38.4–40.8%) (1). Approximately 80,000 women were living with a renal allograft in 2014. One would expect 400 cases (0.5%) of breast cancers from kidney recipients. Our population of 398 KTR with breast cancer, managed to include nearly all the predicted carcinomas despite the low number of cases. This is even more impressive considering NIS only captures 20% of the national inpatient sample.
Next, a crucial piece of information missing from our data was the specific immunosuppressive drug used in each patient. Reports have emphasized over a 3-fold increase in cancer incidence after renal transplant (28) New evidence has identified immunosuppression as the primary cause behind the increase (29). Tacrolimus is generally regarded as the preferred immunosuppressant after KT (30). But it has been associated with increased incidence of malignancy compared to cyclosporine-based drugs, that pose greater wound healing complications (31,32).
Another limitation was the lack of information regarding neoadjuvant treatment for breast cancer prior to surgical intervention. Previous chemotherapeutic intervention can influence the incidence of post-operative complications. Several groups have already quantified the impact of neoadjuvant therapy on breast cancer surgery. One analysis concluded that 18% of patients experienced major complications (33). Another discovered that adverse events are more frequent in cases of mastectomy with immediate reconstruction (34). Presumably those on immunosuppressants and receiving neoadjuvant chemotherapy would be even more susceptible to complications.
We identified increased rates of acute renal failure in KTR post-operatively. However, our methodology did not allow access to any kidney function test parameters such as GFR, Creatinine, or urinalysis. Finally, the lack of long-term data on recovery, graft function and failure were major drawbacks of the project. Nonetheless, this is the largest analysis of the outcomes of breast cancer surgery in KTR and we identified several novel findings.
Conclusions
In this retrospective analysis, we identified that KTR underwent lumpectomy or mastectomy to treat breast carcinoma at a younger age but struggled with more comorbidities compared to the cohort. A higher proportion were African American and Hispanic women. Women with a renal allograft suffered nearly double the rate of complications and were more likely to need pRBC and fresh frozen plasma transfusions compared to nontransplant recipients. More KTR underwent breast surgery at a TC. TC demonstrated superior outcomes compared to other hospitals. Patients experienced fewer complications, and shorter post-operative stays at TCs.
This report advances the understanding of the unique risk factors of KTR undergoing breast surgery. Decreased morbidity at TCs is likely attributable to more experience in anticipating the needs of KTR and responding with a coordinated multidisciplinary approach to complicated cancer care. Our data supports the growing evidence that advocates for increased surgical treatment of KTR at TCs. We aim to inform future physician decision making in the treatment of transplant recipients. Although we establish the benefit of treatment at TC, further investigations of treatment methods at TCs are necessary to establish best practice guidelines for KTR.
Acknowledgments
David Samson of Westchester Medical Center for assistance with data gathering and analysis.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-56
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-56). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Data - OPTN. Accessed January 21, 2020. Available online: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/
- Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Kidney. Am J Transplant 2019;19:19-123. [Crossref] [PubMed]
- Engels EA, Pfeiffer RM, Fraumeni JF, et al. Spectrum of Cancer Risk Among US Solid Organ Transplant Recipients. JAMA 2011;306:1891-901. [Crossref] [PubMed]
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019;69:438-51. [Crossref] [PubMed]
- Rossetto A, Tulissi P, De Marchi F, et al. De Novo Solid Tumors After Kidney Transplantation: Is It Time for a Patient-Tailored Risk Assessment? Experience From a Single Center. Transplant Proc 2015;47:2116-20. [Crossref] [PubMed]
- Apel H, Walschburger-Zorn K, Häberle L, et al. De novo malignancies in renal transplant recipients: experience at a single center with 1882 transplant patients over 39 yr. Clin Transplant 2013;27:E30-E36. [Crossref] [PubMed]
- Miao Y, Everly JJ, Gross TG, et al. De Novo Cancers Arising in Organ Transplant Recipients are Associated With Adverse Outcomes Compared With the General Population. Transplantation 2009;87:1347-59. [Crossref] [PubMed]
- Buell JF, Hanaway MJ, Trofe J, et al. De novo breast cancer in renal transplant recipients. Transplant Proc 2002;34:1778-9. [Crossref] [PubMed]
- Kwak HY, Chae BJ, Bae JS, et al. Breast cancer after kidney transplantation: a single institution review. World J Surg Oncol 2013;11:77. [Crossref] [PubMed]
- DiBrito SR, Haugen CE, Holscher CM, et al. Complications, Length of Stay, and Cost of Cholecystectomy in Kidney Transplant Recipients. Am J Surg 2018;216:694-8. [Crossref] [PubMed]
- DiBrito SR, Olorundare IO, Holscher CM, et al. Surgical Approach, Cost, and Complications of Appendectomy in Kidney Transplant Recipients. Clin Transplant 2018;32:e13245 [Crossref] [PubMed]
- DiBrito SR, Alimi Y, Olorundare IO, et al. Outcomes Following Colorectal Resection in Kidney Transplant Recipients. J Gastrointest Surg 2018;22:1603-10. [Crossref] [PubMed]
- USCS Data Visualizations. Accessed January 21, 2020. Available online: https://gis.cdc.gov/grasp/USCS/DataViz.html
- Oetting WS, Schladt DP, Guan W, et al. Genomewide Association Study of Tacrolimus Concentrations in African American Kidney Transplant Recipients Identifies Multiple CYP3A5 Alleles. Am J Transplant 2016;16:574-82. [Crossref] [PubMed]
- Baez Y, Giron F, Niño-Murcia A, et al. Experience With Alemtuzumab (Campath-1H) as Induction Agent in Renal Transplantation Followed by Steroid-Free Immunosuppression. Transplant Proc 2008;40:697-9. [Crossref] [PubMed]
- Elixhauser A, Steiner C, Harris D, et al. Comorbidity Measures for Use with Administrative Data. Med Care 1998;36:8-27. [Crossref] [PubMed]
- van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009;47:626-33. [Crossref] [PubMed]
- Molnar MZ, Novak M, Ambrus C, et al. Anemia in kidney transplanted patients. Clin Transplant 2005;19:825-33. [Crossref] [PubMed]
- Mix TCH, Kazmi W, Khan S, et al. Anemia: A Continuing Problem Following Kidney Transplantation. Am J Transplant 2003;3:1426-33. [Crossref] [PubMed]
- Zellner E, Lentz R, Chuang C, et al. Complications Following Plastic Surgery in Solid Organ Transplant Recipients: A Descriptive Cohort Study. J Aesthetic Reconstr Surg 2016. doi:
10.4172/2472-1905.100019 .10.4172/2472-1905.100019 - Vitug AF, Newman LA. Complications in Breast Surgery. Surg Clin North Am 2007;87:431-51. [Crossref] [PubMed]
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78:285-91. [Crossref] [PubMed]
- Koonce SL, Giles B, McLaughlin SA, et al. Breast Reconstruction After Solid Organ Transplant. Ann Plast Surg 2015;75:343-7. [Crossref] [PubMed]
- Whiting J. Perioperative concerns for transplant recipients undergoing nontransplant surgery. Surg Clin North Am 2006;86:1185-1194. vi-vii. [Crossref] [PubMed]
- Gill JS, Wright AJ, Delmonico FL, et al. Towards Improving the Transfer of Care of Kidney Transplant Recipients. Am J Transplant 2017;17:54-9. [Crossref] [PubMed]
- Burke LG, Frakt AB, Khullar D, et al. Association Between Teaching Status and Mortality in US Hospitals. JAMA 2017;317:2105-13. [Crossref] [PubMed]
- v2 CH7 Transplantation. Accessed March 6, 2020. Available online: https://www.usrds.org/2016/view/v2_07.aspx
- Vajdic CM, McDonald SP, McCredie MRE, et al. Cancer Incidence Before and After Kidney Transplantation. JAMA 2006;296:2823-31. [Crossref] [PubMed]
- Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59-67. [Crossref] [PubMed]
- Webster AC, Woodroffe RC, Taylor RS, et al. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ 2005;331:810. [Crossref] [PubMed]
- Knoll GA, Kokolo MB, Mallick R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ 2014;349:g6679. [Crossref] [PubMed]
- Schäffer M, Schier R, Napirei M, et al. Sirolimus impairs wound healing. Langenbecks Arch Surg 2007;392:297-303. [Crossref] [PubMed]
- Garvey EM, Gray RJ, Wasif N, et al. Neoadjuvant therapy and breast cancer surgery: a closer look at postoperative complications. Am J Surg 2013;206:894-8. [Crossref] [PubMed]
- Decker MR, Greenblatt DY, Havlena J, et al. Impact of Neoadjuvant Chemotherapy on Wound Complications after Breast Surgery. Surgery 2012;152:382-8. [Crossref] [PubMed]
Cite this article as: Choubey AP, Parsikia A, Dubchuk C, Castaldi M, Latifi R, Ortiz J. Renal transplant recipients suffer significantly more complications but not mortality after breast cancer surgery and benefit from treatment at transplant centers. Ann Breast Surg 2021;5:13.

