
Perspectivising tumescent mastectomy: innovation in preserving mastectomy skin flap perfusion—a narrative review
Introduction
Oncologic breast surgery has changed towards breast conserving surgery and neoadjuvant chemotherapy. Despite this, mastectomy is still offered to 1/4–1/3 of all breast cancer women. It is used for extensive or multifocal disease in the breast and is offered in risk-reducing surgery to women with genetic high risk of breast cancer.
The most important issue in mastectomies is the oncologic safety. This requires first of all adequately resection of the breast parenchyma including any diagnosed pathologic findings.
Secondly, mastectomy has to be done with a minimum risk of complications, in order to avoid postponing possible adjuvant therapy, but also to ensure quick recovery and optimized aesthetic result. Frequent complications to mastectomy are skin flap necrosis and infection. A large meta-analysis showed risk of this in direct to implant breast reconstruction on 8.6% and 7.8%, respectively and the ultimate failure—implant loss in 14.4% (1). To avoid these, skin flap perfusion is crucial.
Aesthetic result is essential in both simple mastectomy and when mastectomy is accompanied with breast reconstruction—either immediate or delayed and either implant based or autologous. The thicker subcutaneous coverage of a silicone implant, the more natural and aesthetic pleasant result. The following article is presented in accordance with the Narrative Review reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-4/rc).
Oncological safety and breast margins
Performing an oncological safe mastectomy includes removal of all breast tissue, leaving no or a minimum of residual breast tissue in the mastectomy margins (2,3). Residual Breast tissue is especially undesirable in therapeutic mastectomies where radiotherapy is not always included in the adjuvant therapy as with breast conserving surgery. Also in the genetic high risk population, residual breast tissue is unwanted. After risk-reducing mastectomy this group are often no longer offered breast cancer screening, why residual tissue adds additional occult risk. Skin sparring mastectomy in this group can be done with or without sparring the nipple areola complex and associated with either autologous or implant based immediate breast reconstruction. Performing skin sparring mastectomy implies keeping all or most of the skin above the breast parenchyma. This results in leaving a larger resection margin in the patient than with simple mastectomy where an elliptical skin coverage of the breast is resected with the breast tissue. It is therefore even more important that the surgeon is aware of the right dissection plane in order to minimize the residual breast tissue in the skin flap.
An understanding of the anatomy of the breast is prerequisite for avoiding residual breast tissue.
Most agree that the fascia over the pectoral muscle delimits the breast tissue profound. Concerns in interest are: (I) superficial margins in order to find the right clivage between breast tissue and skin flaps and (II) periphery boundaries of the breast tissue in the cranial, lateral, caudal, and medial directions.
Superficial breast margins
Figure 1 shows a slice of breast reduction specimen microscopically. One can see the skin on top consisting of epidermis and dermis overlying a subcutaneous layer of fatty tissue. In the center, stretching through the subcutaneous fatty layer and reaching the skin is a Coopers ligament with glandular tissue. Removing all breast tissue implies removing the majority of Coopers ligaments.
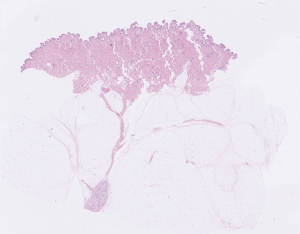
Most breast surgeons find that there exists a superficial macroscopically identifiable oncoplastic plane or dissection plane. This plane separates the breast parenchyma including at-risk duct with the overlying subcutaneous fat and dermis compositing the skin flaps. The dissection should follow this plane. The plane varies in identifiability among women and within the breast. The plane has traditionally been found by incising the skin and subcutaneous tissue and then with counter traction applied to the underlying breast and the skin flaps has consecutively been dissected.
A superficial fascia layer of the body, consisting of connective tissue network between the subdermal planes to the underlying muscle fascia, has been described (4). Controversies regarding the existence of a corresponding superficial fascia in the breast exists. There have been several anatomical studies investigating if a superficial fascia equivalent to the dissection plane exists in the breast (5). A study by Muntan et al. found that the superficial fascia layer divides in two layers with the mammary gland in between (6). Beer et al. studied breast reduction specimens in 62 breasts and found absence of a superficial fascia in 44% (7). In the group with a microscopically identifiable superficial fascia this was often not detectable macroscopically. Microscopically, however, it contained islands of breast tissue in 42% but no breast tissue above the fascia in the skin flaps. The distance from this superficial fascia to the dermis was greatly variable but very little in the majority of the women and the authors argued, that following this, would not leave vital skin flaps behind. No breast tissue above the fascia and a wide variability in the distance from the superficial fascia to the dermis was corroborated by Larson et al. (8) Furthermore, they found that this distance was not associated with BMI, age or the weight of the breast specimen.
Although newer anatomical studies have revealed interesting evidence of a three dimensional system of a subcutaneous fascia (9), it seems that there is great variability in the presence of a macroscopic detectable superficial layer among women and in distance of this to the skin. It is therefore unpredictable, useless in dissection of the skin flaps and probably not the same as the dissection plane noted by surgeons.
Peripheral breast boundaries
The anatomical boundaries have been described as from the second or third to the sixth or seventh rib inferiorly and from the midaxillary line to the lateral border of the sternum (10). Furthermore, the breast tissue frequently extends into the axilla as the axillary tail of Spence.
As this sounds clearly defined, it seems that the peripheral border is not easily found by the surgeon. Studies find not only residual breast tissue in the skin flaps but also in the periphery including inframammary fold, the infraclavicular region, the axillary tail and especially the upper parasternal region and lower outer quadrant (2,11). Residual tissue depends on the surgeon’s expertise, thus every surgeon should evaluate mastectomy quality and comprehensiveness in a close cooperation with his or her pathologist (12).
Vascular anatomy of the breast skin and nipple-areola complex
Even the most elegant mastectomy with or without primary reconstruction is doomed to fail if the overlying skin suffer from necrosis and planed adjuvant therapy is postponed (13).
During mastectomy, a large undermining is done and all blood flow from beneath penetrating through the mammary gland is removed. The skin flap survival is primarily dependent on the blood flow originating from the periphery where the skin flap is attached to the thorax. The blood supply derives from the subdermal plexus and the subcutaneous vessels that are extensions from the intercostal perforators. These vessels lie in the subdermal layer of the mastectomy flaps hence superficial to the dissection plane. Optimizing the blood supply in the skin flaps depends majorly on two essential principles: (I) atraumatic technique in order to minimize injuries in the subdermal plexus during mastectomy, (II) sparring the perforators from the internal mammary artery lateral to the sternum by careful dissection around these.
Tumescent mastectomy technique
Tumescence with epinephrine has been used for local anesthesia for ages and increasingly during the last decade for subcutaneous mastectomy. The technique includes infiltration of epinephrine containing solution with a blunt cannula in the entire breast between the glandular tissue and the skin in the subcutaneous fatty tissue. Using epinephrine results in contractions of the small blood vessels and decreased bleeding enabling a better overview of the surgical field. The technique is described in details and visualized with video previously (14). After infiltration, the mastectomy can be done blindly with blunt dissection or under visual guidance.
When the blind technique is used, the Metzenbaum scissor is simply moved back and forth in the entire breast area with the opposite hand on top of the breast to immobilize the skin flaps and sense the movement of the scissor. This should be as unhindered as possible with only the cutting of Coopers ligament (ligamentum suspensorium mammae) as obstacles in the movement. A longer pair of scissors can be an advantage when inframammary incision is used in nipple sparring subcutaneous mastectomy.
The visual mastectomy dissection technique is done with Metzenbaum scissor and consists of two movements; first, blunt dissection where the two branches of the pair of scissors is separated along the breast tissue detaching the fat lobules from the glandular tissue between the Coopers ligaments. Then, as shown in Figure 2 the ligaments are cut with the pair of scissors by a sliding movement towards the top of the ligaments to release these from their attachment towards the dermis. The cut has to be as close to the skin as possible in order to remove possible glandular tissue within the ligaments.
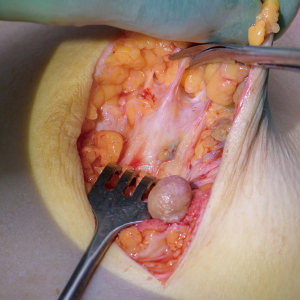
The blind technique reduces the surgery time significantly and it is easier for the surgeon. The visual guided technique, which is preferred by the author, has on the other hand several advantages.
First, the epinephrine solution enhances the visual differentiation of glandular tissue including Coopers ligaments from the subcutaneous fat. These structures become much more visible and it is easy to the surgeon to refine the dissection to keep the fatty tissue on the skin flaps and remove if not all, then as close as possible to all glandular tissue increasing the oncological safety. This is clearly shown in Figure 2. Where the yellow fat on the underside of the skin flaps is easily differentiated from the white glandular tissue beneath.
Secondly, the atraumatic technique in the right avascular dissection plane conserves the blood vessels in the dermal layer and ensures optimal blood supply of the skin flaps. The survival of the skin flaps depends on the blood flow to the skin. Keeping this unharmed greatly reduces the risk of postsurgical complications including necrosis, and wound dehiscence but also infections, since the risk of infection is increased if the blood flow to the skin is compromised.
And third, it makes preservation of most of the subcutaneous fatty tissue possible. A thick layer of fatty tissue enables a cosmetic superior result. This is especially important when the mastectomy is immediately followed by breast reconstruction with silicone implants but also ensures an optimal result using autologous flaps. A thick coverage results in a far softer natural and aesthetic acceptable result than thin skin flaps which visualize the implant edges in an unnatural way. Furthermore, a thick skin flap implies a longer distance from the skin surface to the implant, reducing risk of infection.
Risks associated with tumescent has been reported. A meta-analysis based on 4049 breasts from 5 studies with Level of Evidence III suggested increased risk of skin flap necrosis with tumescent mastectomy technique (15). Stratification into blind and visual technique was not done. This was not corroborated in a later published study with Level of Evidence I by Lautrup et al. (16) They randomized 371 breasts to either tumescent mastectomy technique or mastectomy with electrocautery technique. They found no statistically significant difference regarding necrosis, infection, or bleeding. These patients were mastectomized using the blind dissection technique in the tumescent group and extra attention to preserve the blood supply to the skin has therefore not been given. Ng et al. reviewed nipple sparing mastectomy and compared necrosis among women having mastectomy with either tumescence and sharp dissection or electrocautery (17). They found statistically significant higher frequencies of both full thickness necrosis (12.8% vs. 1.3%) and partial thickness necrosis (33.3% vs. 13.0%) among the electrocautery group compared with the tumescent group. Other studies including both autologous and implant based immediate breast reconstruction find neither increased nor decreased risk of skin flap necrosis with the tumescent technique (18-21). These studies do not, however, specify whether blind or visual technique has been used. Surgical time for tumescent technique has been shown to be equal (16) or shorter than compared techniques (17,19).
Indocyanine green laser angiography (ICG) is a modality widely used to describe intraoperative flap perfusion. This has been adapted to mastectomy skin flaps especially when immediate breast reconstruction is planned. Failure to detect perfusion problems may result in postoperative necrosis, reoperation, infection and ultimately implant loss (22). Usage of ICG intraoperative empowers the reconstructive surgeon to detect areas with low perfusion and followed with immediate excision of critically perfused areas before reconstruction, reduces the risk of post-operative necrosis and frequency of reoperation (23,24). Furthermore, ICG- angiography has been shown to be superior to clinical judgement (25).
Using ICG along with tumescent mastectomy technique has, however, been shown to be complicated. Typically, a low score of perfusions is found. When left in situ without excision, the tumescent skin flap does not subsequently suffer from necrosis as predicted by the ICG. It seems therefore not advisable to combine ICG with tumescent technique. Indocyanine green laser angiography, however, do not improve perfusion of mastectomy skin flaps. Neither does it prevent nor reduce risk of necrosis, it just visualizes low perfused tissue areas susceptible to necrosis (26-28).
Even if the differentiation between glandular tissue and fatty tissue is more clearly visualized and makes it possible to also include most of the Coopers ligament in the resected tissue, this technique does not exclude the risk of residual breast tissue completely. Karusseit et al. demonstrated small islands of breast tissue in the subcutaneous fatty tissue (29). Some of these might represent breast tissue in cross section of Coopers ligaments, but some might also just be naturally dispersed islands of breast tissue. While the cautious dissection technique described here would eliminate most of the Coopers ligaments it would not eliminate such possible tissue islands located in the subcutaneous fatty tissue. Therefore, the existence of small amount of residual breast tissue cannot be ruled out. The author has, however, on several occasions resected some of the dissected skin flaps, when these were in abundance, and had the pathologist to especially go through this for identification of residual breast tissue. This has not been found in the histologic examination. A more systematic examination of residual breast tissue in dissected skin flaps after tumescent mastectomy remains to be done.
Conclusions
Tumescent mastectomy technique used under the guidance of vision reduces bleeding and thereby enhances visualization of the correct dissection plane. The technique optimizes removal of breast tissue including Coopers ligaments and seems therefore to optimize oncologic safety. It is furthermore a less traumatic technique sparing the subcutaneous fatty tissue and the subdermal layer of blood vessels optimizing skin flap perfusion and thereby possibly reducing the risk of skin necrosis. Intraoperative use of indocyanine green laser angiography to assess tissue perfusion in real time is, however, invalid when tumescent technique has been used. If skin resection and choice of implant in immediate breast reconstruction depend on this, tumescent technique needs to be avoided.
Acknowledgments
The author wishes to acknowledge Anne Marie Bak Jylling, MD, Research Unit of Pathology, Department of Clinical Research, University of Southern Denmark and Department of Pathology, Odense University Hospital for kindly supplying with Figure 1.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard and Jørn Bo Thomsen) for the series “Breast Reconstruction - The True Multidisciplinary Approach” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-4/rc
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-4/coif). The series “Breast Reconstruction - The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. CB serves as an unpaid editorial board member of Annals of Breast Surgery from August 2021 to July 2023. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Obtainment of the illustration used in this paper were in accordance with the ethical standards of the national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Basta MN, Gerety PA, Serletti JM, et al. A Systematic Review and Head-to-Head Meta-Analysis of Outcomes following Direct-to-Implant versus Conventional Two-Stage Implant Reconstruction. Plast Reconstr Surg 2015;136:1135-44. [Crossref] [PubMed]
- Griepsma M, de Roy van Zuidewijn DB, Grond AJ, et al. Residual breast tissue after mastectomy: how often and where is it located? Ann Surg Oncol 2014;21:1260-6. [Crossref] [PubMed]
- Ustun I, Beksac K, Kandemir O, et al. Location and Frequency of Residual Breast Tissue after Mastectomy. Breast Care (Basel) 2019;14:212-5. [Crossref] [PubMed]
- Lockwood TE. Superficial fascial system (SFS) of the trunk and extremities: a new concept. Plast Reconstr Surg 1991;87:1009-18. [Crossref] [PubMed]
- Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg 2014;101:899-911. [Crossref] [PubMed]
- Muntan CD, Sundine MJ, Rink RD, et al. Inframammary fold: a histologic reappraisal. Plast Reconstr Surg 2000;105:549-56; discussion 557. [Crossref] [PubMed]
- Beer GM, Varga Z, Budi S, et al. Incidence of the superficial fascia and its relevance in skin-sparing mastectomy. Cancer 2002;94:1619-25. [Crossref] [PubMed]
- Larson DL, Basir Z, Bruce T. Is oncologic safety compatible with a predictably viable mastectomy skin flap? Plast Reconstr Surg 2011;127:27-33. [Crossref] [PubMed]
- Rehnke RD, Groening RM, Van Buskirk ER, et al. Anatomy of the Superficial Fascia System of the Breast: A Comprehensive Theory of Breast Fascial Anatomy. Plast Reconstr Surg 2018;142:1135-44. [Crossref] [PubMed]
- Lemaine V, Simmons PS. The adolescent female: Breast and reproductive embryology and anatomy. Clin Anat 2013;26:22-8. [Crossref] [PubMed]
- Giannotti DG, Hanna SA, Cerri GG, et al. Analysis of Skin Flap Thickness and Residual Breast Tissue After Mastectomy. Int J Radiat Oncol Biol Phys 2018;102:82-91. [Crossref] [PubMed]
- Papassotiropoulos B, Guth U, Chiesa F, et al. Prospective Evaluation of Residual Breast Tissue After Skin- or Nipple-Sparing Mastectomy: Results of the SKINI-Trial. Ann Surg Oncol 2019;26:1254-62. [Crossref] [PubMed]
- Frey JD, Salibian AA, Choi M, et al. The Importance of Tissue Perfusion in Reconstructive Breast Surgery. Plast Reconstr Surg 2019;144:21S-9S. [Crossref] [PubMed]
- Bille C, Dalaei F, Thomsen JB. Identifying the dissection plane for mastectomy-description and visualization of our technique. Gland Surg 2019;8:S276-80. [Crossref] [PubMed]
- Siotos C, Aston JW, Euhus DM, et al. The Use of Tumescent Technique in Mastectomy and Related Complications: A Meta-Analysis. Plast Reconstr Surg 2019;143:39-48. [Crossref] [PubMed]
- Lautrup MD, Thomsen JB, Christensen RD, et al. Tumescent technique versus electrocautery mastectomy: A randomized controlled trial. Surg Oncol 2020;34:276-82. [Crossref] [PubMed]
- Ng T, Knowles S, Brackstone M, et al. Mastectomy flap necrosis after nipple-sparing mastectomy and immediate implant-based reconstruction: An evaluation of tumescence and sharp dissection technique on surgical outcomes. Breast J 2019;25:1079-83. [Crossref] [PubMed]
- Abbott AM, Miller BT, Tuttle TM. Outcomes after tumescence technique versus electrocautery mastectomy. Ann Surg Oncol 2012;19:2607-11. [Crossref] [PubMed]
- Gipponi M, Baldelli I, Atzori G, et al. Tumescent Anesthesia in Skin- and Nipple-sparing Mastectomy: Results of a Prospective Clinical Study. Anticancer Res 2017;37:349-52. [Crossref] [PubMed]
- Khavanin N, Fine NA, Bethke KP, et al. Tumescent technique does not increase the risk of complication following mastectomy with immediate reconstruction. Ann Surg Oncol 2014;21:384-8. [Crossref] [PubMed]
- Vargas CR, Koolen PG, Ho OA, et al. Tumescent mastectomy technique in autologous breast reconstruction. J Surg Res 2015;198:525-9. [Crossref] [PubMed]
- Pruimboom T, Schols RM, Van Kuijk SM, et al. Indocyanine green angiography for preventing postoperative mastectomy skin flap necrosis in immediate breast reconstruction. Cochrane Database Syst Rev 2020;4:CD013280. [Crossref] [PubMed]
- da Silva Neto E, Figueiredo PHM, Moro MG, et al. Use of laser-assisted indocyanine green angiography in breast reconstruction: Systematic review and meta-analysis. J Surg Oncol 2020;121:759-65. [PubMed]
- Moyer HR, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plast Reconstr Surg 2012;129:1043-8. [Crossref] [PubMed]
- Alstrup T, Christensen BO, Damsgaard TE. ICG angiography in immediate and delayed autologous breast reconstructions: peroperative evaluation and postoperative outcomes. J Plast Surg Hand Surg 2018;52:307-11. [Crossref] [PubMed]
- Mlodinow AS, Fine NA, Khavanin N, et al. Risk factors for mastectomy flap necrosis following immediate tissue expander breast reconstruction. J Plast Surg Hand Surg 2014;48:322-6. [Crossref] [PubMed]
- Munabi NC, Olorunnipa OB, Goltsman D, et al. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg 2014;67:449-55. [Crossref] [PubMed]
- Xue EY, Schultz JJ, Therattil PJ, et al. Indocyanine Green Laser Angiography in the Setting of Tumescence. Eplasty 2019;19:e1. [PubMed]
- Karusseit VO, Oberholzer HM, Irsigler NG, et al. Determination of the accuracy of juxtacapsular dissection of the breast. What is left behind? Int J Surg 2014;12:384-9. [Crossref] [PubMed]
Cite this article as: Bille C. Perspectivising tumescent mastectomy: innovation in preserving mastectomy skin flap perfusion—a narrative review. Ann Breast Surg 2022;6:5.

