
The versatile latissimus dorsi flap: old and reliable or outmoded—with or without an add on?
Introduction
Today’s plastic surgeons and post-mastectomy patients are collaborating closely on reconstructive options. In fact, breast reconstruction has become an integral facet on the breast cancer treatment algorithm. Plastic surgeons have several reconstructive methods to choose from. Traditionally, the myocutaneous latissimus dorsi (LD) flap has been considered one of the workhorse flaps for autologous breast reconstruction. It is a reliable reconstructive option with a consistent vascularity, and it is easy to learn as no microvascular anastomosis is needed (1,2). The LD can be used in both immediate and delayed settings (1), in partial breast reconstructions (3-6), in uni- or bilateral cases (7-9), together with implants or expanders, or as an autologous flap—alone or with fat grafting (10).
The LD flap was first described by Iginio Tansini. In 1896, he published a dorsal cutaneous flap to cover the defect after breast cancer surgery, and in 1906 he redesigned the procedure to include the LD muscle in the flap (11-13). His method, a radical mastectomy with the LD flap, was popular throughout Europe between 1910 and 1920 (11). The flap was reintroduced for breast reconstruction in the 1970’s (14-16). In northern Europe, Finland has been one of the forerunners using the LD flap (17). This paper is a narrative review of the different aspects of using the LD flap for breast reconstruction.
Variations of the LD flap
Numerous variations and refinements to the conventional LD flap exist. Some modifications, such as the extended LD, aim to add volume by including fat extensions above or below the muscle, for example the subcutaneous, lumbar and subserratal fat, and parascapular and scapula “fat fascia” (1,18-21). In addition, a fleur-de-lis skin paddle version has been used to carry additional fat on the surface of the LD muscle (22). Other variations aim to decrease donor site morbidity, such as the muscle sparing versions (23-26) and the thoracodorsal artery perforator (TAP) flap (27), to name a few. In addition, endoscopic (28-32) and robotic (33,34) LD muscle harvesting has been introduced.
When to consider an LD?
Although free flaps have overtook pedicled flaps as the primary autologous reconstruction modality, there are cases when a microsurgical reconstruction is not suitable or available. For example, the lack of other suitable soft tissue, comorbidities, obesity, smoking, prior major abdominal surgery, or unavailability of microsurgical services advocate other autologous reconstructive modalities (10,35,36). In these cases, the LD flap offers a good option. In addition, fat grafting has given this traditional flap a new resurgence in popularity as the primary total autologous reconstructive method (10).
Use of the LD flap is also relative to cultural beliefs and geographical constraints. In some countries, the LD has gone almost extinct and is mainly saved for tertiary or palliative purposes, such as for irradiated patients, delayed reconstructions or for the salvage after failed primary or secondary reconstruction (37,38). In other countries, however, the LD is part of the standard repertoire. The LD flap is a good option when microsurgical techniques are not available. In some countries, few patients have access to a practicing microsurgeon (39). Furthermore, a survey showed that only one fourth of practicing US plastic surgeons perform any microsurgical breast reconstruction (40). In addition, the proportion of post mastectomy non-autologous, implant-based reconstructions have grown in the US, whereas the number of autologous reconstructions generally have declined (41). This is, in part, due to the increase in the number of contralateral prophylactic mastectomies, and may, in part, reflect the reimbursement trends (41). Patient education and awareness, leading to a fear of adverse effects in the donor sites, may also contribute to the decline in the use of the LD.
Obesity and the LD
Obesity is considered a risk factor for extensive surgery, including microsurgery. Convincing meta-analyses have shown a clear increase in overall complications, recipient and donor-site complications, and partial flap failure in patients with a body mass index (BMI) ≥30 kg/m2 (42). According to the recent studies, the LD flap seems to be a safer option in overweight patients. Yezhelyev et al. evaluated the influence of BMI on the complications after postmastectomy LD flap reconstruction (43). They concluded that the incidence of both flap and donor site complications after LD reconstruction was not significantly different in overweight (BMI 25–29.9 kg/m2) and obese (BMI ≥30 kg/m2) patients compared to the normal weight population. However, obese patients were more likely to develop mastectomy skin flap necrosis (43). In addition, Novak et al. compared complication rates between immediately fat-grafted LD and free tissue transfer in obese (BMI ≥30 kg/m2) population, and found out that the free tissue transfer group had a significantly higher rate of major and systemic complications (44).
Although LD reconstruction appears to be a safer option for overweight patients, one could assume that in breast reconstruction surgeries of approximately the same duration and recovery, the same systemic complications tend to be present—microsurgery or not. Currently, in our own practice, immediate or delayed elective LD-based breast reconstruction, is not recommended for patients with a BMI of 30 or more.
Donor site sequalae
The harvest of the LD flap comes with some drawbacks. The contour deformity of the back after the harvest, together with a long and visible scar, may be undesirable by some (2,45). Seroma formation in the back is the most common complication (46). It is treated with a prolonged suction drainage followed by outpatient aspirations after the drain has been removed (1). To prevent this problem, different solutions have been attempted. A recent prospective randomized controlled trial compared the efficacy of fibrin glue, triamcinolone acetonide, and quilting sutures in the seroma prevention after LD reconstruction. This study showed that the use of quilting sutures significantly decreases the incidence of donor-site seromas, leads to earlier drain removal and maintains a low complication profile (46).
The shoulder-related donor site morbidity and the extent of its severity is debated. The literature on this subject is quite controversial (47-49). Some state that the effect of the LD harvest on the shoulder function is negligible and minimal, whereas others have found that the impairment of the function is significant (47). A recent systematic review and meta-analysis of functional shoulder impairment after LD breast reconstruction, including 26 articles published until 5/2017, concluded that although the LD flap transfer appears to affect shoulder function, these limitations seem to be minimal. However, many of the studies comprised of small series, and some had a rather short follow-up period. Thus, the authors stated that the existing literature on the long-term shoulder function impairment is insufficient to draw any firm conclusions (47). Lohana et al. studied the functional recovery after bilateral extended autologous latissimus dorsi (EALD) breast reconstruction (50). They stated that bilateral EALD breast reconstruction does not appear to cause significant long-term impairment of shoulder function. However, they concluded that women should be appropriately counselled and preoperatively screened, and intensive physiotherapy might be needed.
With regard to the LD flap types, it seems that sparing the LD muscle can result in less functional implications than other types of LD flaps used (47-49). A recent prospective randomized controlled trial compared shoulder function after delayed breast reconstruction by either a LD flap or a TAP flap with assessment at baseline and 3, 6, and 12 months postoperatively. The study showed that patient-reported shoulder-related pain was significantly lower in the TAP group at 12 months after surgery when adjusting for pain at baseline, and the patients had better function of the shoulder 1 year after the reconstruction (51). In addition, patients reconstructed with the LD flap had a higher level of shoulder related pain and a reduced ability to perform normal daily functions while the range of movement and the strength of the shoulder did not seem to be influenced significantly. However, they stated that a longer follow-up period is needed to establish whether the observed difference change with time.
Should we cut the thoracodorsal nerve?
Optimal management of the thoracodorsal nerve in pedicled LD flaps for mastectomy reconstruction is controversial. Animation deformity due to contraction of the muscle may cause a functional and aesthetic problem as well as be distressing for patients (1). To solve this issue, division of the thoracodorsal nerve has been proposed. However, flap denervation has been suggested to cause muscle atrophy leading to poor soft tissue coverage of a possible implant. Kääriäinen et al. challenged the idea that the resection of the nerve leads automatically to a volume loss and protects from pain and untoward muscle movement (52,53). Histology of the LD flaps with resected nerves showed that muscle atrophy was replaced with fatty degeneration 1 year post operatively while the volume of the flap was preserved on magnetic resonance imaging (MRI). Furthermore, patients with denervated LD flaps presented with a variety of animation and pain symptoms. The authors concluded that for the interest of operation time and simplicity, there is no need to cut the nerve. However, the possibility of the nerve resection having been too distal in the study subjects was not discussed. A recent retrospective clinical and anatomical study strongly suggests that reanimation does eventually occur despite nerve transection and is often symptomatic. Persistent late onset animation deformity is attributable to anatomical differences in the thoracodorsal branching patterns, rather than patient (age, BMI, smoking) or the therapeutic (oncology or surgery related) factors (54). Other studies may have failed to monitor this, due to short- or nonsystematic follow-up of patients. The thoracodorsal nerve starts to branch about 4 cm proximal to the superior border of the LD muscle and thus careful dissection of the nerve branch as proximally as deemed safe has been recommended. As this is technically arduous, preoperative counselling of the patient that dynamic motion may return years postoperatively is advised (54).
Does the LD flap need an add-on?
To ensure sufficient size, the LD flap was traditionally quite routinely combined with implants or expanders, representing the classical use of the LD myocutaneous flap (Figure 1). During the last decades this practice has been questioned due to implant-related complications, such as infection, extrusion, periprosthetic contraction, rupture, and more recently, the suggested association with the anaplastic large cell lymphoma (55,56). Multiple operations related to the implant-based problems seemed overwhelming for the patient and the healthcare system.
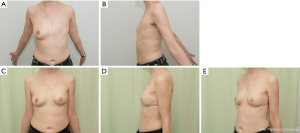
Improved technical skills and equipment have led to large volume fat grating (57) resulting in a sufficient, and in many cases predictable, take rate (58). This has increased the use of fat grafting not only for full breast reconstruction (57,59,60), but also for aesthetic augmentation and, for this review interestingly, as an add on to the LD flap. With the LD flap, free fat grafting can be inserted into the muscular and the subcutaneous part of the flap, into the chest wall surface, under the dermis, and especially into the pectoralis major muscle (Figure 2, Video 1). In our series covering the last 10 years, fewer implants have been used as an add on to the LD flap, but fat grafting has become a more frequent adjunct. The fat grafting can be done either during the LD reconstruction (Figures 2,3) or at a later timepoint (Video 1).
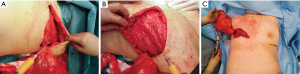
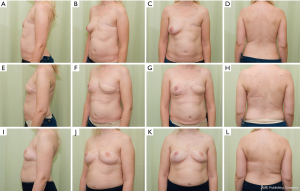
In a study by Leuzzi et al. the number and the type of revision procedures, duration of the hospitalization, the complication rate, and the patient satisfaction were evaluated in a retrospective cohort of patients undergoing LD reconstruction, either with an add on of an implant or with fat augmentation. Patient satisfaction was assessed using the patient-reported outcomes instrument BREAST-Q. Findings concerning the total hospitalization time, overall duration of the reconstruction process, and the distribution of supplementary surgical procedures demonstrated no statistically significant differences between the implant and the fat grafting groups. However, patients in the fat grafting group scored higher in the satisfaction with breast domain of the BREAST-Q. Leuzzi et al. concluded that the addition of a breast implant with LD reconstruction does not decrease the breast reconstruction time in terms of number of the revision procedures and hospitalization time, yet exposes patients to a higher complication rate and does not improve patient satisfaction (56). This was supported by Demiri et al., who stated that the fat augmented LD flap constitutes an alternative method for delayed autologous reconstruction after post-mastectomy irradiation, avoiding implant-related complications (55). In other studies, the automatic use of an implant as an add on is hardly questioned (51).
Can contralateral reduction mammaplasty promote health?
Despite appropriate patient selection, extensive flap harvesting, and either fat- or implant enhancement of the LD reconstruction, a massive breast cannot be achieved. In patients with a hypertrophic contralateral breast, opting for a unilateral LD reconstruction, a symmetrizing reduction mammaplasty has several health promoting effects. Cancer survivors undergoing delayed breast reconstruction may have benefited from oncoplastic surgery or a contralateral breast reduction at the time of the mastectomy. However, the option of a contralateral procedure can be considered also at this later stage. Reduction mammaplasty as such rehabilitates neck-, shoulder- and back-related straining problems (61). Notably, even in healthy, non-cancer subjects, abnormal histopathological findings are revealed in 10% of the patients; of the findings 1% are malignant and 5.5% are high-risk lesions (62). In patients with breast cancer, the figures double (63). Therefore, histopathological analysis of the specimens should be thoroughly considered.
Cost-effectiveness analysis for breast reconstruction; where does the LD flap stand?
Cost-effectiveness analysis guides evidence-based practices of plastic surgeons by quantifying the balance between the risks and the benefits of each treatment strategy from both a patient perspective and a provider perspective (64). If the provider is a public health care facility, the number and the duration of reconstructive procedures, and, above all, the durability of the result plays a major role. On the contrary, if the provider is a private business driven by insurance or industry influences, multiple procedures over the years may give a better profit, enhanced by reimbursement strategies (41). Interestingly, the cost effectiveness analysis on five widely used breast reconstruction techniques clearly favored autologous reconstruction in both radiated and non-radiated patients. In more detail, the pedicled autologous tissue reconstruction was slightly more cost-effective than the free autologous tissue options in both cohorts (64).
Nevertheless, patient centered solutions should be based on the validated patient-reported outcome measures (PROM), available for breast surgeons in many languages to date, so that decision making can be based on solid data (65). For the patient, every surgery is an investment in time and an exposure to morbidity. Thus, cost-efficiency analyses widely applicable to most institutions should be used with prudency, and they are not to be enforced over individual patient preferences (64).
Conclusions
The LD flap is still a worthy choice for breast reconstruction in a selected group of patients, especially when other alternatives are not available. Even in good hands, the LD reconstruction warrants thorough patient counselling and information, as some untoward consequences may appear at a later stage. The implant add-on is associated with the potential of further implant-related sequalae. Fat grafting for flap augmentation and scar correction has resurrected the LD flap as a versatile tool for breast reconstruction.
Acknowledgments
The professional skills and friendly help of our AV-technician Tero Hanski and language editor Pauliina Homsy are greatly appreciated.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jørn Bo Thomsen and Tine Engberg Damsgaard) for the series “Breast reconstruction - The True Multidisciplinary Approach” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-29/coif). The series “Breast reconstruction - The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hammond DC. Latissimus dorsi flap breast reconstruction. Clin Plast Surg 2007;34:75-82; abstract vi-vii. [Crossref] [PubMed]
- Hammond DC. Latissimus dorsi flap breast reconstruction. Plast Reconstr Surg 2009;124:1055-63. [Crossref] [PubMed]
- Losken A, Hamdi M. Partial breast reconstruction: current perspectives. Plast Reconstr Surg 2009;124:722-36. [Crossref] [PubMed]
- Rainsbury RM. Breast-sparing reconstruction with latissimus dorsi miniflaps. Eur J Surg Oncol 2002;28:891-5. [Crossref] [PubMed]
- Hamdi M, Wolfli J, Van Landuyt K. Partial mastectomy reconstruction. Clin Plast Surg 2007;34:51-62; abstract vi. [Crossref] [PubMed]
- Hamdi M. Oncoplastic and reconstructive surgery of the breast. Breast 2013;22:S100-5. [Crossref] [PubMed]
- Smith BK, Cohen BE, Biggs TM, et al. Simultaneous bilateral breast reconstruction using latissimus dorsi myocutaneous flaps: a retrospective review of an institutional experience. Plast Reconstr Surg 2001;108:1174-81; discussion 1182. [Crossref] [PubMed]
- Chiaramonte MF, Nahabedian MY. Bilateral breast reconstruction with the latissimus dorsi musculocutaneous flap: the importance of patient positioning. Ann Plast Surg 2001;46:163-6. [Crossref] [PubMed]
- Losken A, Nicholas CS, Pinell XA, et al. Outcomes evaluation following bilateral breast reconstruction using latissimus dorsi myocutaneous flaps. Ann Plast Surg 2010;65:17-22. [Crossref] [PubMed]
- Mushin OP, Myers PL, Langstein HN. Indications and Controversies for Complete and Implant-Enhanced Latissimus Dorsi Breast Reconstructions. Clin Plast Surg 2018;45:75-81. [Crossref] [PubMed]
- Maxwell GP. Iginio Tansini and the origin of the latissimus dorsi musculocutaneous flap. Plast Reconstr Surg 1980;65:686-92. [Crossref] [PubMed]
- Tansini I. Sopra il mio nuovo precesso de amputazione della mamella. Riforma Medica 1906;12:757.
- Tansini I. Nuovo processo per l'amputazione della mammaella per cancre. Reforma Medica 1896;12:3.
- Schneider WJ, Hill HL, Brown RG. Latissimus dorsi myocutaneous flap for breast reconstruction. Br J Plast Surg 1977;30:277-81. [Crossref] [PubMed]
- Olivari N. The latissimus flap. Br J Plast Surg 1976;29:126-8. [Crossref] [PubMed]
- Bostwick J, Vasconez LO, Jurkiewicz MJ. Breast reconstruction after a radical mastectomy. Plast Reconstr Surg 1978;61:682-93. [Crossref] [PubMed]
- Peltoniemi H, Asko-Seljavaara S, Härmä M, et al. Latissimus dorsi breast reconstruction. Long term results and return of sensibility. Scand J Plast Reconstr Surg Hand Surg 1993;27:127-31. [Crossref] [PubMed]
- Germann G, Steinau HU. Breast reconstruction with the extended latissimus dorsi flap. Plast Reconstr Surg 1996;97:519-26. [Crossref] [PubMed]
- Chang DW, Youssef A, Cha S, et al. Autologous breast reconstruction with the extended latissimus dorsi flap. Plast Reconstr Surg 2002;110:751-9; discussion 760. [Crossref] [PubMed]
- Hokin JA. Mastectomy reconstruction without a prosthetic implant. Plast Reconstr Surg 1983;72:810-8. [Crossref] [PubMed]
- Hokin JA, Silfverskiold KL. Breast reconstruction without an implant: results and complications using an extended latissimus dorsi flap. Plast Reconstr Surg 1987;79:58-66. [Crossref] [PubMed]
- McGraw J, Papp C. Latissimus dorsi myocutaneous flap: "Fleur de lis" reconstruction. In: Hartrampf CR. Editor. Breast Reconstruction with Living Tissue. Norfolk, Va: Hampton Press, 1991.
- Schwabegger AH, Harpf C, Rainer C. Muscle-sparing latissimus dorsi myocutaneous flap with maintenance of muscle innervation, function, and aesthetic appearance of the donor site. Plast Reconstr Surg 2003;111:1407-11. [Crossref] [PubMed]
- Hamdi M, Van Landuyt K, Monstrey S, et al. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg 2004;57:531-9. [Crossref] [PubMed]
- Brackley PT, Mishra A, Sigaroudina M, et al. Modified muscle sparing latissimus dorsi with implant for total breast reconstruction - extending the boundaries. J Plast Reconstr Aesthet Surg 2010;63:1495-502. [Crossref] [PubMed]
- Hamdi M, Van Landuyt K, Hijjawi JB, et al. Surgical technique in pedicled thoracodorsal artery perforator flaps: a clinical experience with 99 patients. Plast Reconstr Surg 2008;121:1632-41. [Crossref] [PubMed]
- Angrigiani C, Grilli D, Siebert J. Latissimus dorsi musculocutaneous flap without muscle. Plast Reconstr Surg 1995;96:1608-14. [Crossref] [PubMed]
- Friedlander L, Sundin J. Minimally invasive harvesting of the latissimus dorsi. Plast Reconstr Surg 1994;94:881-4. [Crossref] [PubMed]
- Fine NA, Orgill DP, Pribaz JJ. Early clinical experience in endoscopic-assisted muscle flap harvest. Ann Plast Surg 1994;33:465-9; discussion 469. [Crossref] [PubMed]
- Pomel C, Missana MC, Atallah D, et al. Endoscopic muscular latissimus dorsi flap harvesting for immediate breast reconstruction after skin sparing mastectomy. Eur J Surg Oncol 2003;29:127-31. [Crossref] [PubMed]
- Kiiski J, Kaartinen I, Kotaluoto S, et al. Modified approach for endoscopic harvest of the latissimus dorsi free flap with CO insufflation and standard laparoscopic equipment. Microsurgery 2017;37:383-7. [Crossref] [PubMed]
- Cho BC, Lee JH, Ramasastry SS, et al. Free latissimus dorsi muscle transfer using an endoscopic technique. Ann Plast Surg 1997;38:586-93. [Crossref] [PubMed]
- Selber JC, Baumann DP, Holsinger FC. Robotic latissimus dorsi muscle harvest: a case series. Plast Reconstr Surg 2012;129:1305-12. [Crossref] [PubMed]
- Selber JC. Robotic latissimus dorsi muscle harvest. Plast Reconstr Surg 2011;128:88e-90e. [Crossref] [PubMed]
- Macadam SA, Bovill ES, Buchel EW, et al. Evidence-Based Medicine: Autologous Breast Reconstruction. Plast Reconstr Surg 2017;139:204e-229e. [Crossref] [PubMed]
- Seidenstuecker K, Munder B, Mahajan AL, et al. Morbidity of microsurgical breast reconstruction in patients with comorbid conditions. Plast Reconstr Surg 2011;127:1086-92. [Crossref] [PubMed]
- DeLong MR, Tandon VJ, Rudkin GH, et al. Latissimus Dorsi Flap Breast Reconstruction-A Nationwide Inpatient Sample Review. Ann Plast Surg 2017;78:S185-8. [Crossref] [PubMed]
- Hammond DC, Simon AM, Khuthaila DK, et al. Latissimus dorsi flap salvage of the partially failed TRAM flap breast reconstruction. Plast Reconstr Surg 2007;120:382-9. [Crossref] [PubMed]
- Kaur N, Gupta A, Saini S. Breast reconstruction in low resource settings: Autologous latissimus dorsi flap provides a viable option. Indian J Cancer 2015;52:291-5. [Crossref] [PubMed]
- Kulkarni AR, Sears ED, Atisha DM, et al. Use of autologous and microsurgical breast reconstruction by U.S. plastic surgeons. Plast Reconstr Surg 2013;132:534-41. [Crossref] [PubMed]
- Panchal H, Matros E. Current Trends in Postmastectomy Breast Reconstruction. Plast Reconstr Surg 2017;140:7S-13S. [Crossref] [PubMed]
- Schaverien MV, Mcculley SJ. Effect of obesity on outcomes of free autologous breast reconstruction: a meta-analysis. Microsurgery 2014;34:484-97. [Crossref] [PubMed]
- Yezhelyev M, Duggal CS, Carlson GW, et al. Complications of latissimus dorsi flap breast reconstruction in overweight and obese patients. Ann Plast Surg 2013;70:557-62. [Crossref] [PubMed]
- Novak MD, Blough JT, Abraham JT, et al. Breast Reconstruction in Obese Patients: The Fat Grafted Latissimus versus Abdominal Free Tissue Transfer. Plast Reconstr Surg Glob Open 2020;8:e2668. [Crossref] [PubMed]
- Bailey S, Saint-Cyr M, Zhang K, et al. Breast reconstruction with the latissimus dorsi flap: women's preference for scar location. Plast Reconstr Surg 2010;126:358-65. [Crossref] [PubMed]
- Hart AM, Duggal C, Pinell-White X, et al. A Prospective Randomized Trial of the Efficacy of Fibrin Glue, Triamcinolone Acetonide, and Quilting Sutures in Seroma Prevention after Latissimus Dorsi Breast Reconstruction. Plast Reconstr Surg 2017;139:854e-863e. [Crossref] [PubMed]
- Steffenssen MCW, Kristiansen AH, Damsgaard TE. A Systematic Review and Meta-analysis of Functional Shoulder Impairment After Latissimus Dorsi Breast Reconstruction. Ann Plast Surg 2019;82:116-27. [Crossref] [PubMed]
- Lee KT, Mun GH. A systematic review of functional donor-site morbidity after latissimus dorsi muscle transfer. Plast Reconstr Surg 2014;134:303-14. [Crossref] [PubMed]
- Blackburn NE, Mc Veigh JG, Mc Caughan E, et al. The musculoskeletal consequences of breast reconstruction using the latissimus dorsi muscle for women following mastectomy for breast cancer: A critical review. Eur J Cancer Care (Engl) 2018;27:e12664. [Crossref] [PubMed]
- Lohana P, Button J, Young D, et al. Functional recovery after bilateral extended autologous latissimus dorsi breast reconstruction: A prospective observational study. J Plast Reconstr Aesthet Surg 2019;72:1060-6. [Crossref] [PubMed]
- Rindom MB, Gunnarsson GL, Lautrup MD, et al. Shoulder-related donor site morbidity after delayed breast reconstruction with pedicled flaps from the back: An open label randomized controlled clinical trial. J Plast Reconstr Aesthet Surg 2019;72:1942-9. [Crossref] [PubMed]
- Kääriäinen M, Giordano S, Kauhanen S, et al. No need to cut the nerve in LD reconstruction to avoid jumping of the breast: a prospective randomized study. J Plast Reconstr Aesthet Surg 2014;67:1106-10. [Crossref] [PubMed]
- Kääriäinen M, Giordano S, Kauhanen S, et al. The significance of latissimus dorsi flap innervation in delayed breast reconstruction: a prospective randomized study-magnetic resonance imaging and histologic findings. Plast Reconstr Surg 2011;128:637e-645e. [Crossref] [PubMed]
- Senger JL, Wolfli J. Late animation deformity in the denervated pedicled latissimus dorsi flap. Breast J 2020;26:685-90. [Crossref] [PubMed]
- Demiri EC, Dionyssiou DD, Tsimponis A, et al. Outcomes of Fat-Augmented Latissimus Dorsi (FALD) Flap Versus Implant-Based Latissimus Dorsi Flap for Delayed Post-radiation Breast Reconstruction. Aesthetic Plast Surg 2018;42:692-701. [Crossref] [PubMed]
- Leuzzi S, Stivala A, Shaff JB, et al. Latissimus dorsi breast reconstruction with or without implants: A comparison between outcome and patient satisfaction. J Plast Reconstr Aesthet Surg 2019;72:381-93. [Crossref] [PubMed]
- Khouri RK, Smit JM, Cardoso E, et al. Percutaneous aponeurotomy and lipofilling: a regenerative alternative to flap reconstruction? Plast Reconstr Surg 2013;132:1280-90. [Crossref] [PubMed]
- Peltoniemi HH, Salmi A, Miettinen S, et al. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: a prospective comparative study. J Plast Reconstr Aesthet Surg 2013;66:1494-503. [Crossref] [PubMed]
- Kauhanen S, Höckerstedt A. Full breast reconstruction with fat and how to recycle the "dog-ear". Gland Surg 2019;8:S297-300. [Crossref] [PubMed]
- Hoppe DL, Ueberreiter K, Surlemont Y, et al. Breast reconstruction de novo by water-jet assisted autologous fat grafting--a retrospective study. Ger Med Sci 2013;11:Doc17. [PubMed]
- Singh KA, Losken A. Additional benefits of reduction mammaplasty: a systematic review of the literature. Plast Reconstr Surg 2012;129:562-70. [Crossref] [PubMed]
- Merkkola-von Schantz PA, Jahkola TA, Krogerus LA, et al. Should we routinely analyze reduction mammaplasty specimens? J Plast Reconstr Aesthet Surg 2017;70:196-202. [Crossref] [PubMed]
- Merkkola-von Schantz PA, Jahkola TA, Krogerus LA, et al. Reduction mammaplasty in patients with history of breast cancer: The incidence of occult cancer and high-risk lesions. Breast 2017;35:157-61. [Crossref] [PubMed]
- Grover R, Padula WV, Van Vliet M, et al. Comparing five alternative methods of breast reconstruction surgery: a cost-effectiveness analysis. Plast Reconstr Surg 2013;132:709e-723e. [Crossref] [PubMed]
- Pusic AL, Matros E, Fine N, et al. Patient-Reported Outcomes 1 Year After Immediate Breast Reconstruction: Results of the Mastectomy Reconstruction Outcomes Consortium Study. J Clin Oncol 2017;35:2499-506. [Crossref] [PubMed]
Cite this article as: Merkkola-von Schantz PA, Kauhanen MSC. The versatile latissimus dorsi flap: old and reliable or outmoded—with or without an add on? Ann Breast Surg 2022;6:13.

