
Validation of international predictive nomograms for non-sentinel lymph node metastases in Hong Kong breast cancer patients with positive sentinel lymph nodes
Introduction
Breast cancer is the most common cancer in women and its incidence in Hong Kong has doubled over the past 10 years, resulting in a great health burden. One in 15 women develop breast cancer over the course of their lifetime (1). In 2016 alone, 4,132 patients were diagnosed with breast cancer and 704 patients died from it, accounting for 12.2% of all cancer related deaths in women.
Axillary lymph node status is one of the most important prognostic predictors. Hence, accurate axillary staging is of paramount importance in treatment planning. Over the past decades, breast cancer treatment has been evolving with a paradigm shift towards “less is more”, particularly evident in the de-escalation of axillary surgery. With the publication of the NSABP B32 trial (2), routine axillary lymph node dissection (ALND) is now out of favour and replaced by sentinel lymph node biopsy (SLNB) in clinically node negative patients for axillary staging. The ASOCOG Z0011 trial also suggests that ALND can be omitted in selected patients with positive sentinel lymph nodes (SLNs) undergoing breast conservation treatment with no detrimental effect on their 10-year overall survival and locoregional recurrence risk (3,4). Though the role of ALND has been on the decline (5,6), for patients not fulfilling the Z0011 criteria, ALND is still the standard treatment. Yet, up to 40–70% of these patients do not have further metastases in their non-SLNs (NSLNs) after axillary dissection (7,8); and they are exposed to a risk of lymphedema of up to 25–40%, arm paraesthesia up to 30%, pain and reduced shoulder movement (9,10).
Can there be a way to predict which patient would truly benefit from an axillary dissection? Several nomograms have been developed with the aim of predicting the risk of non-sentinel lymph metastasis, including the Memorial Sloan Kettering Cancer Center (MSKCC) (11), the MD Anderson Cancer Center (MDACC) nomogram (12), Tenon (13), Stanford (14), Cambridge (15), etc. Though these nomograms have been developed in Caucasian countries, multiple validation studies have been performed in predominantly Asian populations such in Korea (16), Japan (17,18), Taiwan (19), Singapore (20) and China (Mainland) (21). The results of these studies are summarized in Table 1.
Table 1
| Authors [date of publication] | Region | Duration | Number of patients | NSLN metastases (%) | Number of patients with macrometastases in SLN (%) | Nomograms | AUROC |
|---|---|---|---|---|---|---|---|
| Kuo et al. [2013] | Taiwan | 1999–2011 | 324 | 88 (27.2) | Not mentioned | MSKCC | 0.738 |
| Chue et al. [2014] | Singapore | 2004–2009 | 266 | 147 (55.3) | Not mentioned | MSKCC | 0.716 |
| Wu et al. [2018] | China | 2010–2016 | 236 | 105 (44.5) | 224 (94.9) | MSKCC | 0.677 |
| Tenon | 0.673 | ||||||
| Sasada et al. [2012] | Japan | 2000–2009 | 116 | 53 (46.0) | Not mentioned | MSKCC | 0.730 |
| Tanaka et al. [2013] | Japan | 2002–2010 | 89 | 31 (34.8) | 59 (66.2) | MSKCC | 0.701 |
| Cho et al. [2008] | Korea | 2004–2007 | 82 | 39 (47.6) | Not mentioned | MSKCC | 0.786 |
| MDACC | 0.691 | ||||||
| Tenon | 0.751 |
MSKCC, Memorial Sloan Kettering Cancer Center; MDACC, MD Anderson Cancer Center; NSLN, non-sentinel lymph node; SLN, sentinel lymph node; AUROC, area under the receiver operating characteristic curve.
Hong Kong breast cancer patients have an earlier age of onset compared with their western counterparts, and the highest 5-year relative survival rate amongst Asian countries (22). Hence, deciding on optimal axillary management is crucial. This study represents the first study in Hong Kong validating MSKCC, MDACC and Tenon scores on prediction of NSLN metastases, with additional subgroup analysis on patients with minimal axillary disease burden. We selected these nomograms for validation in our patients as they are easily accessible and easy to calculate, facilitating its use in the frontline for risk assessment, patient communication and individualization of treatment.
We present the following article in accordance with the TRIPOD reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-20/rc).
Methods
This is a retrospective study of patients with primary breast cancer who received surgery between April 2011 and April 2019 in Queen Elizabeth Hospital, Hong Kong, China. Patients are eligible for inclusion into this study if they fulfilled these criteria: (I) clinically node negative with no distant metastasis on diagnosis; (II) underwent ipsilateral successful SLNB with frozen section positive for metastasis (macrometastases or micrometastases); (III) received axillary dissection. Patients were excluded if they (I) were diagnosed to have solely ductal carcinoma in situ; (II) had axillary lymph node metastases or distant metastases on presentation; (III) received neoadjuvant chemotherapy; (IV) were recurrence of previous breast cancer.
Demographic data including age, gender, diagnostic investigations, operative details, tumour attributes [grade, lymphovascular invasion, size, hormone receptor and human epidermal growth factor receptor 2 (HER2) status], details of SLNB, axillary dissection and their results were collected.
From the collected data, predicted risk of NSLN metastases was calculated from the following websites:
- MSKCC predictive results were calculated from the online calculator at http://nomograms.mskcc.org/Breast/BreastAdditionalNonSLNMetastasesPage.aspx;
- MDACC results were analysed according to the calculator at http://www3.mdanderson.org/app/medcalc/bc_nomogram2/index.cfm?pagename=nsln.
Tenon score was calculated from combination of three parameters with reference to the system pioneered by Barranger et al. (13): (I) presence of macrometastases in SLNs gives a score of 2; otherwise it gives 0. (II) A histological tumour size of more than 2 cm gives a score of 3; 1.1–2 cm gives a score of 1.5; and less than or equal to 1 cm gives a score of 0. (III) If the proportion of involved sentinel nodes among all sentinel nodes is 1, then the score is 2; those between 0.5 to 1 gives a score of 1 and less than 0.5 gives 0. Patients with a combined score of 3.5 or less had a 97.3% chance of being free from NSLN metastases.
Statistical analyses
Statistical analyses were performed with SPSS version 23. The accuracy of the MSKCC nomogram, MDACC, and Tenon scores in predicting NSLN metastases was assessed by the receiver operating characteristic (ROC) curve analysis. A greater area under the ROC curve (AUROC) equals superior concordance between predicted and observed outcomes. Multivariate analysis by logistic regression was performed to determine the independent predictors of NSLN metastases in our patient population. A P value of <0.05 represented statistical significance.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research has been approved by Hong Kong Kowloon Central cluster research ethics committee (Ref: KC/KE-19-0134/ER-3). Informed consent has been waived by the ethics committee as this retrospective research poses no risk to patients.
Results
A total of 1,545 patients received breast surgery in the study period, of which 126 patients were eligible for inclusion. A flowchart of patient recruitment is shown in Figure 1. Their demographics and tumour characteristics are summarized in Table 2.
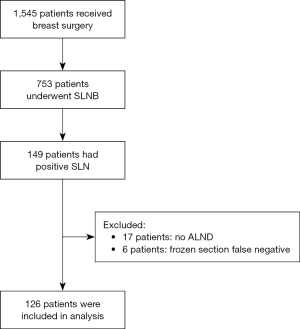
Table 2
| Characteristics | Number of patients (%) |
|---|---|
| Age (mean) | 56.73 |
| Gender (female) | 126 (100.0) |
| Type of operation | |
| Mastectomy | 88 (69.8) |
| BCT | 38 (30.2) |
| T stage | |
| T1 | 52 (41.3) |
| T2 | 65 (51.6) |
| T3 | 9 (7.1) |
| T4 | 0 (0.0) |
| Grade (modified Bloom and Richardson) | |
| 1 | 26 (20.6) |
| 2 | 59 (46.9) |
| 3 | 41 (32.5) |
| Lymphovascular invasion | |
| Present | 68 (55.3) |
| Absent | 55 (44.7) |
| Multifocal | |
| Yes | 25 (19.8) |
| No | 101 (80.2) |
| Type of tumour | |
| Invasive ductal | 113 (89.7) |
| Invasive lobular | 3 (2.4) |
| Others | 10 (7.9) |
| ER status | |
| Positive | 100 (79.4) |
| Negative | 26 (20.6) |
| PR status | |
| Positive | 90 (71.4) |
| Negative | 36 (28.6) |
| HER2 status | |
| Positive | 20 (16.3) |
| Negative | 102 (82.9) |
| Equivocal | 1 (0.8) |
| Method of SLN localization | |
| Dye | 24 (19.0) |
| Isotope | 82 (65.1) |
| Both | 20 (15.9) |
| Number of SLN (median) | 4 (range, 1–13) |
| Number of positive SLN (median) | 1 (range, 1–4) |
| Type of positive SLN | |
| Macrometastases | 96 (76.2) |
| Micrometastases | 30 (23.8) |
| Positive NSLN present | 35 (27.8) |
| Number of positive NSLN (median) | 1 (range, 1–9) |
| N stage | |
| 1mi | 23 (18.3) |
| 1 | 87 (69.0) |
| 2 | 15 (11.9) |
| 3 | 1 (0.8) |
| Extranodal spread | |
| Present | 37 (31.1) |
| Absent | 82 (68.9) |
BCT, breast conserving treatment; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; SLN, sentinel lymph node; NSLN, non-sentinel lymph node.
The majority of patients had early-stage disease, only 7.1% (9 patients) had T3 disease. SLNs were predominantly localized with isotope (65.1%) and supplemented with blue dye (15.9%) if localization was deemed poor on lymphoscintigraphy. The majority of positive SLNs were macrometastases (76.2%). Only 35 patients out of 126 (27.8%) had subsequent positive NSLNs after axillary dissection, and amongst these patients, the majority had only 1–2 further positive NSLNs.
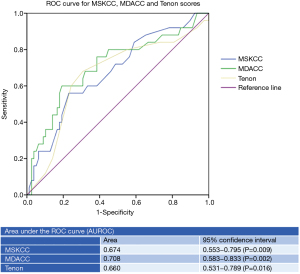
From this demographic data, ROC analysis was performed and the AUROC of MSKCC, MDACC and Tenon scores are shown in Figure 2. The AUROC of MDACC (0.708, 95% CI: 0.583–0.833) was the highest, followed by MSKCC (0.674, 95% CI: 0.553–0.795) and Tenon (0.660, 95% CI: 0.531–0.789). Further subgroup analysis performed after exclusion of patients with only micrometastases in their SLNs showed major improvement in the AUROC of MDACC (0.745, 95% CI: 0.635–0.855, P<0.005) and MSKCC (0.701, 95% CI: 0.584–0.819, P=0.003). The AUROC of Tenon score remained similar (0.656, 95% CI: 0.540–0.773, P=0.021). Subgroup analysis of patients with only one positive SLN found that all three nomograms showed poor performance for predicting NSLN metastases. The AUROC of MDACC (0.618, 95% CI: 0.457–0.779, P=0.21) was still the highest, compared with MSKCC (0.597, 95% CI: 0.453–0.741, P=0.21) and Tenon score (0.529, 95% CI: 0.368–0.690, P=0.71).
Multivariate analysis performed found grade (OR: 0.107, 95% CI: 0.14–0.801, P=0.03), ratio of positive to negative SLN (OR: 0.005, 95% CI: 0.001–0.639, P=0.033) and extranodal spread (OR: 2.754, 95% CI: 0.979–7.745, P<0.05) as significant independent predictors of NSLN metastases.
Discussion
Accurate assessment of nodal status is paramount in staging and treatment of early breast cancer. Since the introduction of SLNB for breast cancer in 1990s (23), deciding which patients warrant completion axillary dissection has become a conundrum of modern breast surgery. Particularly when the majority of NSLNs are negative, meaning patients are exposed to increased risks of permanent morbidity with no additional benefit.
How do we balance the risk of understaging against overtreating the axilla? In the era of Z0011, axillary dissection is no longer the only standard of care and can be safely omitted in a select group of patients receiving breast conserving surgery. However, there are still lingering concerns with the ASCOG Z0011 trial. Over half of the patients without axillary dissection received “high tangent” radiotherapy which covered the axilla (24). This complicates interpretation of results and raises the possibility that the excellent oncological outcomes may be contributed by incidental axillary irradiation. Subsequent trials designed to address the limitations of Z0011 and to expand its inclusion criteria are currently underway. For example, the UK POSNOC (POsitive Sentinel NOde: adjuvant therapy alone versus adjuvant therapy plus Clearance or axillary radiotherapy) trial (25) and the Holland BOOG 2013-07 trial (26). Both trials have expanded the Z0011 criteria to encompass mastectomy patients randomized to receive either axillary treatment (ALND or radiotherapy) or no treatment.
While we wait for results of these ongoing trials, researchers investigated predictors of NSLN metastases (27-29) hoping to find that magic cut-off to stratify patients to high risk versus low risk of further lymph node metastases. One such study by Hung et al. in Hong Kong (30) identified tumour size less than 3 cm, a single metastatic SLN, micrometastases and absence of extranodal spread as negative predictors for NSLN metastases. Similar results were obtained from our multivariate analysis. We identified grade, positive to negative SLN ratio and presence of extranodal spread as independent predictors of non-sentinel node metastases. Indeed, these parameters are common among formulas developed to predict the risk of NSLN metastases. There are more than 10 similar predictive nomograms developed all over the world, with MDACC, MSKCC and Tenon scores being three of the more heavily researched and validated ones (31).
From our results, all three nomograms show acceptable accuracy in predicting NSLN metastases. Among them, the MDACC calculator is the most accurate with an AUROC of 0.708. This is in line with the results of previous validation studies, which showed an AUROC ranging from 0.58 to 0.79 for MSKCCand 0.706–0.73 (32,33)for MDACC. Some studies have suggested that these nomograms are less accurate for patients with only micrometastases in their sentinel nodes (34,35). Our results indeed concur. Subgroup analysis of patients with only one positive SLN also showed poor predictive accuracy in all three nomograms. This suggests that in patients with minimal axillary disease burden, none of these predictive algorithms are perfect, and certainly not enough to justify omitting axillary dissection just based on their scores.
There are also other limitations of these algorithms. In particular, the MSKCC is only limited for invasive ductal carcinoma and invasive lobular carcinoma and cannot be used on patients with other types of breast cancer like mucinous or papillary carcinoma. In addition, most of the parameters used for calculation such as presence of lymphovascular invasion, exact tumour size, etc. are only available in the post-operative stage. This limits their use in pre-operative counselling and guiding intraoperative decisions.
Yet, despite these limitations, we believe that these nomograms still have a role in clinical practice. This study has validated the use of MDACC, MSKCC and Tenon score in patients in Hong Kong. In our institution, they are currently used to estimate the probability of NSLN metastases in patients who have had false negative SLNs to facilitate counselling on the pros and cons of further axillary treatment. They are also incorporated into oncology protocols to assist decision making during adjuvant radiotherapy planning—regional RT is omitted in a subset of breast cancer patients who did not receive completion ALND with an estimated low risk of further NSLN metastases.
This validation study represents the first of such study conducted in Hong Kong. However, it is limited by the small sample size. Despite including patients over 8 years, only a small proportion of them had positive NSLNs. There is also a selection bias and further limitation in the sample size, as this study spanned across a paradigm shift after the publication of the Z0011 study, resulting in omission of axillary dissection in a number of patients who would have been considered eligible in the initial years of this study but now rendered ineligible for inclusion. There was also some missing data regarding the presence of extranodal spread (total seven patients) before pathology reporting was standardized. Since this information is required for the calculation of MDACC scores, these patients were excluded in the analysis.
Conclusions
In conclusion, MSKCC, MDACC and Tenon scores all show acceptable accuracy in predicting NSLN metastases, but are less accurate in patients with only one positive SLN or micrometastases. MDACC shows the best accuracy in predicting NSLN metastases in our subset of patients in Hong Kong.
Acknowledgments
The authors acknowledge Dr. Cheuk Yi Wong in data collection.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-20/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-20/dss
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-20/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-20/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research has been approved by Hong Kong Kowloon Central cluster research ethics committee (Ref: KC/KE-19-0134/ER-3). Informed consent has been waived by the ethics committee as this retrospective research poses no risk to patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hong Kong Breast Cancer Foundation. Available online: https://www.hkbcf.org/en/breast_health/main/99 (Accessed on 4 April 2019).
- Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11:927-33. [Crossref] [PubMed]
- Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA 2017;318:918-26. [Crossref] [PubMed]
- Giuliano AE, Ballman K, McCall L, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg 2016;264:413-20. [Crossref] [PubMed]
- Jatoi I, Benson JR, Toi M. De-escalation of axillary surgery in early breast cancer. Lancet Oncol 2016;17:e430-41. [Crossref] [PubMed]
- García-Novoa A, Acea-Nebril B, Casal-Beloy I, et al. The decline of axillary lymph node dissection in breast cancer. Evolution of its indication over the last 20 years. Cir Esp 2019;97:222-9. (Engl Ed). [PubMed]
- Goyal A, Douglas-Jones A, Newcombe RG, et al. Predictors of non-sentinel lymph node metastasis in breast cancer patients. Eur J Cancer 2004;40:1731-7. [Crossref] [PubMed]
- Maimaitiaili A, Wu D, Liu Z, et al. Analysis of factors related to non-sentinel lymph node metastasis in 296 sentinel lymph node-positive Chinese breast cancer patients. Cancer Biol Med 2018;15:282-9. [Crossref] [PubMed]
- Del Bianco P, Zavagno G, Burelli P, et al. Morbidity comparison of sentinel lymph node biopsy versus conventional axillary lymph node dissection for breast cancer patients: results of the sentinella-GIVOM Italian randomised clinical trial. Eur J Surg Oncol 2008;34:508-13. [Crossref] [PubMed]
- Rietman JS, Geertzen JH, Hoekstra HJ, et al. Long term treatment related upper limb morbidity and quality of life after sentinel lymph node biopsy for stage I or II breast cancer. Eur J Surg Oncol 2006;32:148-52. [Crossref] [PubMed]
- Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol 2003;10:1140-51. [Crossref] [PubMed]
- Mittendorf EA, Hunt KK, Boughey JC, et al. Incorporation of sentinel lymph node metastasis size into a nomogram predicting nonsentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann Surg 2012;255:109-15. [Crossref] [PubMed]
- Barranger E, Coutant C, Flahault A, et al. An axilla scoring system to predict non-sentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Res Treat 2005;91:113-9. [Crossref] [PubMed]
- Kohrt HE, Olshen RA, Bermas HR, et al. New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer 2008;8:66. [Crossref] [PubMed]
- Pal A, Provenzano E, Duffy SW, et al. A model for predicting non-sentinel lymph node metastatic disease when the sentinel lymph node is positive. Br J Surg 2008;95:302-9. [Crossref] [PubMed]
- Cho J, Han W, Lee JW, et al. A scoring system to predict nonsentinel lymph node status in breast cancer patients with metastatic sentinel lymph nodes: a comparison with other scoring systems. Ann Surg Oncol 2008;15:2278-86. [Crossref] [PubMed]
- Sasada T, Murakami S, Kataoka T, et al. Memorial Sloan-Kettering Cancer Center Nomogram to predict the risk of non-sentinel lymph node metastasis in Japanese breast cancer patients. Surg Today 2012;42:245-9. [Crossref] [PubMed]
- Tanaka S, Sato N, Fujioka H, et al. Validation of online calculators to predict the non-sentinel lymph node status in sentinel lymph node-positive breast cancer patients. Surg Today 2013;43:163-70. [Crossref] [PubMed]
- Kuo YL, Chen WC, Yao WJ, et al. Validation of Memorial Sloan-Kettering Cancer Center nomogram for prediction of non-sentinel lymph node metastasis in sentinel lymph node positive breast cancer patients an international comparison. Int J Surg 2013;11:538-43. [Crossref] [PubMed]
- Chue KM, Yong WS, Thike AA, et al. Predicting the likelihood of additional lymph node metastasis in sentinel lymph node positive breast cancer: validation of the Memorial Sloan-Kettering Cancer Centre (MSKCC) nomogram. J Clin Pathol 2014;67:112-9. [Crossref] [PubMed]
- Wu P, Zhao K, Liang Y, et al. Validation of breast cancer models for predicting the nonsentinel lymph node metastasis after a positive sentinel lymph node biopsy in a Chinese population. Technol Cancer Res Treat 2018;17:1533033818785032. [Crossref] [PubMed]
- Kim Y, Yoo KY, Goodman MT. Differences in incidence, mortality and survival of breast cancer by regions and countries in Asia and contributing factors. Asian Pac J Cancer Prev 2015;16:2857-70. [Crossref] [PubMed]
- Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 1994;220:391-8; discussion 398-401. [Crossref] [PubMed]
- Jagsi R, Chadha M, Moni J, et al. Radiation field design in the ACOSOG Z0011 (Alliance) Trial. J Clin Oncol 2014;32:3600-6. [Crossref] [PubMed]
- Goyal A, Dodwell D. POSNOC: A randomised trial looking at axillary treatment in women with one or two sentinel nodes with macrometastases. Clin Oncol (R Coll Radiol) 2015;27:692-5. [Crossref] [PubMed]
- van Roozendaal LM, de Wilt JH, van Dalen T, et al. The value of completion axillary treatment in sentinel node positive breast cancer patients undergoing a mastectomy: a Dutch randomized controlled multicentre trial (BOOG 2013-07). BMC Cancer 2015;15:610. [Crossref] [PubMed]
- Güven HE, Doğan L, Kültüroğlu MO, et al. Factors influencing non-sentinel node metastasis in patients with macrometastatic sentinel lymph node involvement and validation of three commonly used nomograms. Eur J Breast Health 2017;13:189-93. [Crossref] [PubMed]
- Choi AH, Blount S, Perez MN, et al. Size of extranodal extension on sentinel lymph node dissection in the American College of Surgeons Oncology Group Z0011 Trial Era. JAMA Surg 2015;150:1141-8. [Crossref] [PubMed]
- Kim I, Ryu JM, Kim JM, et al. Development of a nomogram to predict N2 or N3 stage in T1-2 invasive breast cancer patients with no palpable lymphadenopathy. J Breast Cancer 2017;20:270-8. [Crossref] [PubMed]
- Hung WK, Chan MC, Mak KL, et al. Non-sentinel lymph node metastases in breast cancer patients with metastatic sentinel nodes. ANZ J Surg 2005;75:27-31. [Crossref] [PubMed]
- Rouzier R, Uzan C, Rousseau A, et al. Multicenter prospective evaluation of the reliability of the combined use of two models to predict non-sentinel lymph node status in breast cancer patients with metastatic sentinel lymph nodes: the MSKCC nomogram and the Tenon score. Results of the NOTEGS study. Br J Cancer 2017;116:1135-40. [Crossref] [PubMed]
- Dingemans SA, de Rooij PD, van der Vuurst de Vries RM, et al. Validation of six nomograms for predicting non-sentinel lymph node metastases in a Dutch breast cancer population. Ann Surg Oncol 2016;23:477-81. [Crossref] [PubMed]
- Zhu L, Jin L, Li S, et al. Which nomogram is best for predicting non-sentinel lymph node metastasis in breast cancer patients? A meta-analysis. Breast Cancer Res Treat 2013;137:783-95. [Crossref] [PubMed]
- Alran S, De Rycke Y, Fourchotte V, et al. Validation and limitations of use of a breast cancer nomogram predicting the likelihood of non-sentinel node involvement after positive sentinel node biopsy. Ann Surg Oncol 2007;14:2195-201. [Crossref] [PubMed]
- D'Eredità G, Troilo VL, Giardina C, et al. Sentinel lymph node micrometastasis and risk of non-sentinel lymph node metastasis: validation of two breast cancer nomograms. Clin Breast Cancer 2010;10:445-51. [Crossref] [PubMed]
Cite this article as: Wong YY, Kwok KH. Validation of international predictive nomograms for non-sentinel lymph node metastases in Hong Kong breast cancer patients with positive sentinel lymph nodes. Ann Breast Surg 2022;6:11.

