
Osteosarcoma of the breast: a case report of a rare breast malignancy in a patient with prior partial mastectomy
Introduction
Less than 1% of all breast malignancies comprise breast sarcomas, even rarer are primary osteosarcomas of the breast with an incidence of about 12.5% (1). Breast sarcomas arise from non-epithelial parts of the gland, the mesenchymal cells. There is no clear etiology for osteosarcoma of the breast (OSB), however it has been suggested that pre-existing lesions of the breast such as fibroadenomas and phyllodes tumor could lead to malignant transformation or originate from totipotent mesenchymal cells (2,3). At present, no risk factors have been identified; yet, some cases have reported the possibility of radiation therapy or trauma to play a role (2). OSB has a poor prognosis with no optimal treatment algorithm; however, mastectomy without axillary dissection remains an adequate option with or without chemotherapy (2), and in some cases chest wall irradiation due to its high potential for recurrence (4).
We present the following case in accordance with the CARE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-20-104/rc).
Case presentation
This is a 68-year-old G4P4 postmenopausal female with prior history of stage IIB pT2, N1, M0 triple negative right breast cancer, status post partial mastectomy in 2004 and axillary dissection, treated with adjuvant chemotherapy and local radiation with interruption in her chemotherapy due to social reasons. She presented to our clinic in 2018 after referral by oncology for a right palpable breast lesion. Stereotactic biopsy was suggested but she was lost to follow up.
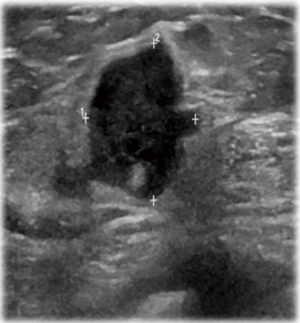
She returned to the clinic again in 2020 after mammography and ultrasonography reported Breast Imaging Reporting and Database System Score 5 (BI-RADS) with 1.43 × 1.37 suspicious lesion in the right breast at 1 o’clock, 9 cm from the nipple (Figure 1). On exam she had a single 2 cm × 2 cm firm to hard nodule at 12 o’clock position in the right breast. There was no discharge or nipple abnormalities noted. She was rescheduled for ultrasound guided right breast biopsy. Pathology results were reported as poorly differentiated malignant neoplasm, favoring poorly differentiated mammary carcinoma with osteoblastic giant cells (Figure 2). Magnetic resonance imaging (MRI) reported an abnormal enhancing 2.2 cm mass in the upper inner left breast consistent with known biopsy proven recurrent or new carcinoma, BI-RADS 6. She was scheduled for a right complete mastectomy. Final pathology was reported as high-grade osteoblastic osteosarcoma (Figures 3-5), deep margin positive for malignancy.
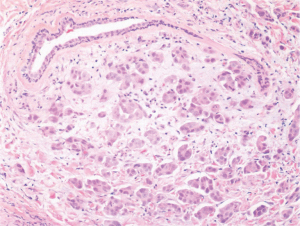
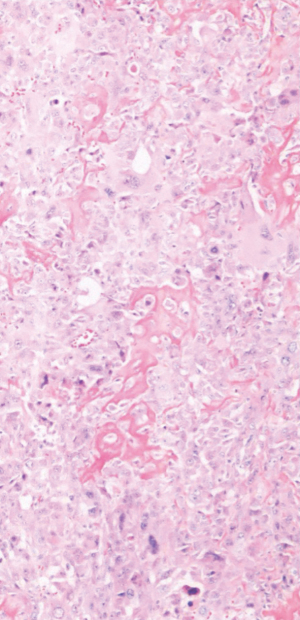
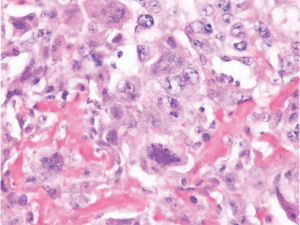
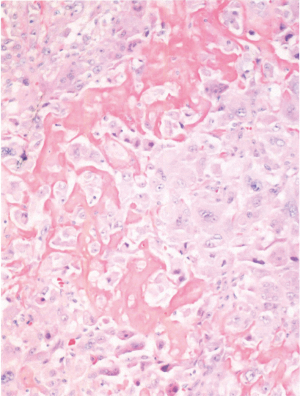
Immunohistochemical characterization of the tumor indicated that the neoplastic cells were diffusely strongly positive with Special AT-rich sequence-binding protein 2 (SATB2) nuclear staining. It was negative for Cytokeratins (CK), low molecular weight, high molecular weight, p63, desmin, transcription factor protein SOX-10 (SOX10), S100 protein, Estrogen Receptor 0%, Progesterone Receptor 0%, HER2 0%, Gross cystic disease fluid protein 15 (GCDFP-15) and mammaglobin (nonspecific haze). Scattered GATA3 positive cell were seen in the tumor. These histological features were typical for what one would find in a tumor showing osteogenic sarcoma differentiation.
The patient was followed by oncology, who recommended Positron Emission Tomography/Computed Tomography (PET/CT). The findings were: operative bed metabolic activity measuring up to 2.7 standard uptake value (SUV), within limits for postoperative change. Bilateral hilar lymph nodes measuring up to 3.8 SUV, possibly reactive. No other abnormal findings on PET/CT were reported. The decision was made for re exploration of the right chest wall and wide local excision of chest wall soft tissue/muscle due to a deep margin positive for malignancy. On exploration, no local recurrence was noted clinically. Part of pectoralis major muscle with subcutaneous fat was removed from the upper chest where the tumor was located. Re-excision margins were negative for malignancy.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
On literature review, most patients with OSB initially presented for a palpable lump of the breast as was seen in our patient. Roughly 25% of patients were complaining of increase in size of the lump (5). Our patient had a mammogram showing a radiolucent mass with benign appearing calcifications in the breast. In 2/3rds of patients, on mammogram a lobulated mass is seen with speckled microcalcifications and the remaining 1/3rd had imaging consistent with fibroadenoma (5). Imaging findings consistent with fibroadenoma is what leads to the undertreatment of osteosarcoma. Imaging (mammogram/ultrasound) remains an important modality in the workup of the disease, however diagnosis can only be made with tissue sampling.
Our patient returned to the operating room for re-excision given a positive deep margin. Norris and Taylor (6) found that it may be necessary to excise the fascia of the pectoralis muscle or even the muscle itself, however, this is only if the mass is seen to invade it. They do not recommend radical mastectomy for mesenchymal tumors of the breast as they feel it is of no benefit to the patient. Instead, they recommended simple mastectomy or wide local resection with or without radiation as there is limited literature to support the use of radiation. Silver and Tavassoli (5) state these tumors have an aggressive nature with propensity towards early recurrence and hematogenous spread with metastases most frequently to the lung with a 5-year survival rate as little as 38%. These findings are consistent with other literature. Our patient presented at age 68 which coincides more with a primary breast osteosarcoma as it typically affects the elderly as opposed to skeletal osteosarcoma which present in the earlier years of life (1). We were also able to rule out a bony origin of the tumor with PET/CT, indicating a breast primary. Also of significance, our patient has prior radiation therapy in the ipsilateral breast. Few cases have been reported of patients developing OSB after exposure of radiotherapy of the breast, indicating that this is likely a risk factor for the disease process (2,5,7)
Although a treatment algorithm does not exist, we recommend surgical excision with wide surgical margins, preferably mastectomy. In the case of positive margins, we recommend re-excision with adequate margins. Lymph node dissection is not indicated when lymphadenopathy is not present, given that sarcomas metastasize hematogenously. In a case series of 20 patients who underwent lymph node dissection, none came back positive for lymph node metastasis (5,7). It remains one of debate whether adjuvant chemotherapy is of any benefit due to the lack of data, however adjuvant chemotherapy does increase survival in patients with osteosarcoma of the bone, for this reason we encourage the use of chemotherapy (3). At present, we do not recommend the use of radiation, given the risk of developing OSB from chest wall irradiation.
Conclusions
There are reported 5 different histologic types: fibroblastic, osteoblastic, chondroblastic, small cell and telangiectatic (8). Our report demonstrated by thorough histological examination and investigation that this was a primary OSB osteoblastic type, a rare malignancy for which case reports are found in the literature. Surgical planning with radiological support is highly recommended. Currently OSB remains an uncommon presentation for breast cancer; several cases have been reported although there are not any treatment guidelines to this day. Intensive reporting of these cases will encourage better association of data for improvement in treatment recommendations. Until then, OSB remains a topic that needs further depth of investigation.
Acknowledgments
We would like to thank Dr. Masooma Niazi, MD, Chairman of Pathology at Bronx Care Health System for the Pathology Slides.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-20-104/rc
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-20-104/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-20-104/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mujtaba B, Nassar SM, Aslam R, et al. Primary Osteosarcoma of the Breast: Pathophysiology and Imaging Review. Curr Probl Diagn Radiol 2020;49:116-23. [Crossref] [PubMed]
- Coussy F, Le Scodan R, Guinebretiere JM, et al. Breast mass with intense 99mTc-diphosphonate uptake revealing primary breast osteosarcoma. J Clin Oncol 2011;29:e428-30. [Crossref] [PubMed]
- Bahrami A, Resetkova E, Ro JY, et al. Primary osteosarcoma of the breast: report of 2 cases. Arch Pathol Lab Med 2007;131:792-5. [Crossref] [PubMed]
- Ogundiran TO, Ademola SA, Oluwatosin OM, et al. Primary osteogenic sarcoma of the breast. World J Surg Oncol 2006;4:90. [Crossref] [PubMed]
- Silver SA, Tavassoli FA. Primary osteogenic sarcoma of the breast: a clinicopathologic analysis of 50 cases. Am J Surg Pathol. 1998;22:925-33. [Crossref] [PubMed]
- Norris HJ, Taylor HB. Sarcomas and related mesenchymal tumors of the breast. Cancer 1968;22:22-8. [Crossref] [PubMed]
- Milne DM, Sookar N, Umakanthan S, et al. Primary Osteosarcoma of the Breast in a Patient Treated Previously for Invasive Ductal Carcinoma: An Unusual Presentation of a Very Rare Primary Breast Malignancy. Case Rep Surg 2020;2020:1594127. [Crossref] [PubMed]
- Trihia H, Valavanis C, Markidou S, et al. Primary osteogenic sarcoma of the breast: cytomorphologic study of 3 cases with histologic correlation. Acta Cytol 2007;51:443-50. [Crossref] [PubMed]
Cite this article as: Hattingh G, Hanssen D, Medina J, Sanghvi M, Shah A. Osteosarcoma of the breast: a case report of a rare breast malignancy in a patient with prior partial mastectomy. Ann Breast Surg 2022;6:19.

