Surgical management of a giant malignant phyllodes tumour of the breast: a case report
Introduction
Phyllodes tumours (PT) of the breast are an uncommon type of fibroepithelial neoplasms, representing 0.5% to 1% of all fibroepithelial neoplasms (1-3). They are classified benign, borderline and malignant tumours, as accorded by the World Health Organization (1-4). They usually present as a large breast tumor with rapid growth above 4 cm, considering those over 10 cm as giant PT (2). Due to its rapid and considerable growth, their diagnosis is challenging with the usual techniques, and core biopsy is necessary in most cases. Their management relies mostly on an adequate surgical resection, taking especial emphasis into achieving resection margins above 1 cm in order to avoid local recurrence. In cases of local recurrence, a new local excision or mastectomy is mandatory. Because of their rapid and accentuated growth, their surgical resection can be technically challenging, considering in large tumours the confection of flaps in order to maintain safe resection margins. Malignant lesions frequently show hematogenous dissemination, regardless of the presence or not of local recurrence (1). Other treatments widely used in breast tumours, such as chemotherapy, radiotherapy or hormone therapy are not routinely used, making it fundamental to provide an adequate excision, with proper margins. We present the following case in accordance with the CARE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-20-150/rc).
Case presentation
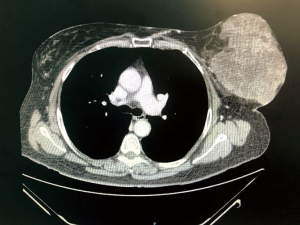
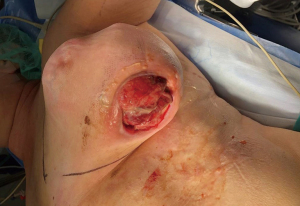
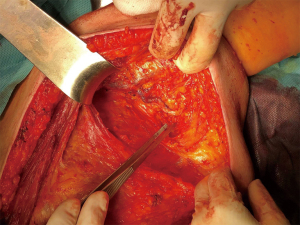
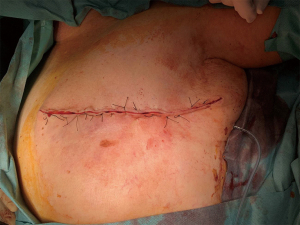
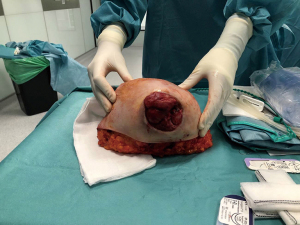
We present the case of a female patient of 62 years old. without any personal or familiar history of breast cancer or any other malignancies, with a rapid growth of a breast mass, over the last month before consultation. She didn’t have any trauma, pain, or infectious symptoms suggestive of other diagnosis. During the physical exam, the breast showed an ulcerated mass of 20 cm in diameter. Local curations of the ulcer were being performed by the patient. A core biopsy was performed under a probable diagnosis of a breast sarcoma. She underwent magnetic resonance and computed tomography (Figure 1), without evidence of any distant metastases, but with positive axillary nodes. After a multidisciplinary discussion in the breast tumour committee, we performed a mastectomy (Figures 2-6). Despite its large size, there was no need to make any advancements flaps, and the pectoral muscle fascia and axillary nodes were dissected to avoid local recurrence. Closed aspirate drains were left in the mastectomy and axillary dissection territories. The patient was discharged without any complications during the early postoperative period. The postoperative diagnosis was of a PT with sarcomatous degeneration, with negative axillary nodes. Our patient is currently on close follow up without local recurrence or distant metastases.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
PT of the breast is an uncommon type of fibroepithelial neoplasms, with less than a 1% of incidence. They typically present an exaggerated intracanalicular pattern with leaf-like fronds protruding into dilated spaces, accompanied by hypercellularity (2). The definition of “phyllodes” originates from the Latin root “Phyllodium” meaning leaf, due to its appearance on microscopy (2,3). They have a variety of behaviors, from benign fibroadenoma to malignant neoplasms with the ability to produce distant metastases.
Usually, the patients show a rapid onset of symptoms with a large, painless mass, as the one described in our case. Due to their large sizes, core needle biopsy or fine needle aspiration may be insufficient for diagnosis. There may be strong similar histological findings, making it difficult to differentiate a benign PT from cellular fibroadenoma, requiring an evaluation of its borders after excisional biopsy (1,2). Both lesions have an increased stromal cellularity, being differentiated by well-circumscribed borders in fibroadenomas and irregular borders in benign PT. On the other hand, malignant PTs must be distinguished from sarcomas and other types of carcinomas. Malignant PTs represent 1/4 of all PTs, having a strong stromal cellularity with cellular atypia, infiltrative borders, mitotic activity of at least 10/10 HPF and a stromal overgrowth with heterologous elements (2-5).
Mastectomy is not always necessary in small lesions, being only recommended when a margin of 1 cm cannot be achieved with a local excision. Positive surgical margins or less than 0.5 cm, recurrent tumours or a diameter over 10 cm—or 5 cm in malignant (6)—have been considered high risk for local recurrence (2,6,7). In patients with benign or borderline tumours with positive margins, is recommended a re-excision or close follow-up with physical examination and ultrasound every 6 months, and annual mammogram (7,8). In malignant lesions with positive margins, a re-excisional surgery would be necessary. Giant PTs represents only 20% of the total of PT and when planning their excision, different reconstructive techniques should be considered, including deep inferior epigastric artery perforator (DIEP), latissimus dorsi or rectus abdominis flaps (9,10). Their large sizes, and the need to achieve a complete excision with safe margins, may require a multidisciplinary approach in terms of reconstruction.
Although an axillary dissection is not routinely performed, 20% of patients with malignant PT have positive nodes (2). Positive nodes during preoperative radiology or physical exam, should orientate the need to complete an axillary dissection. In our case, an axillary dissection was done due to positive preoperative CT, although postoperative specimen of nodes was negative for metastases.
There is no final recommendation regarding radiotherapy. Although it might reduce the rate of local recurrence, a consensus has not been determined due to inconclusive results, leaving the use of postoperative radiotherapy only for determined patients, especially those considered as high risk of recurrence. In those tumours above 5 cm, postoperative radiotherapy should be considered because of the high risk of recurrence (6). After the resection of a recurrent lesion, adjuvant radiotherapy should be considered, following the same principles as soft tissue sarcoma (8).
Adjuvant or neoadjuvant chemotherapy is not routinely used, as there is no evidence of benefit, limiting its use only for compassive indications. Despite the fact that hormone receptors might be positive in up to 75%, hormone therapy is not a recommended treatment in these neoplasms. In cases of metastatic disease, the same recommendations as for soft tissue sarcoma should be considered (8).
A close follow-up is fundamental in these lesions due to its potential of local recurrence. Current NCCN guidelines recommend clinical follow-up for 3 years (8). If there is recurrence without metastasis, re-excision with tumorectomy or mastectomy can be performed without affecting the overall survival. Metastatic disease, mainly in the lung, abdomen and bone decreases the overall survival.
Despite the large size of the tumour we presented, a complete resection with adequate surgical margins was achieved with a mastectomy and without the need of further reconstructive techniques. When treating a PT, the aim to provide negative margins must be warranted, and multidisciplinary surgical strategies planned when needed. Since other adjuvant therapies are not demonstrated to avoid recurrence, all efforts must be towards an adequate resection.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-20-150/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-20-150/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Krings G, Bean GR, Chen YY. Fibroepithelial lesions; The WHO spectrum. Semin Diagn Pathol 2017;34:438-52. [Crossref] [PubMed]
- Yan Z, Gudi M, Lim SH. A large benign phyllodes tumour of the breast: A case report and literature review. Int J Surg Case Rep 2017;39:192-5. [Crossref] [PubMed]
- Strode M. Update on the diagnosis and management of malignant phyllodes tumors of the breast. Breast 2017;33:91-6. [Crossref] [PubMed]
- Hasdemir S, Tolunay Ş, Özşen M, et al. Phyllodes Tumor of the Breast: A Clinicopathological Evaluation of 55 Cases. Eur J Breast Health 2019;16:32-8. [Crossref] [PubMed]
- Lim SZ, Ong KW, Tan BK, et al. Sarcoma of the breast: an update on a rare entity. J Clin Pathol 2016;69:373-81. [Crossref] [PubMed]
- Choi N, Kim K, Shin KH, et al. Malignant and borderline phyllodes tumors of the breast: a multicenter study of 362 patients (KROG 16-08). Breast Cancer Res Treat 2018;171:335-44. [Crossref] [PubMed]
- Ogunbiyi S, Perry A, Jakate K, et al. Phyllodes tumour of the breast and margins: How much is enough. Can J Surg 2019;62:E19-21. [Crossref] [PubMed]
- NCCN Guidelines Version 6 2020 Breast Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Tsuruta Y, Karakawa R, Majima K, et al. The Reconstruction after a Giant Phyllodes Tumor Resection Using a DIEP Flap. Plast Reconstr Surg Glob Open 2020;8:e2760. [Crossref] [PubMed]
- Liang MI, Ramaswamy B, Patterson CC, et al. Giant breast tumors: surgical management of phyllodes tumors, potential for reconstructive surgery and a review of literature. World J Surg Oncol 2008;6:117. [Crossref] [PubMed]
Cite this article as: Guevara-Martínez J, Osorio I, Bernar J, Salido S, Meliga C, Elsner N, Pardo R. Surgical management of a giant malignant phyllodes tumour of the breast: a case report. Ann Breast Surg 2022;6:18.