Current imaging techniques and impact on diagnosis and survival —a narrative review
Introduction
Diagnosis of breast cancer occurs for a woman either during a surveillance imaging programme (breast cancer screening) when there are no symptoms present or in a diagnostic setting when the cancer causes clinical problems. Imaging plays a central role in detection, staging and follow-up. The methods of breast imaging are evolving and their strengths and weaknesses are re-evaluated constantly to formulate recommendations and guidelines beneficial for clinical practice. In this review we summarize the data from current literature, guidelines and emerging research and discuss advantages as well as possible pitfalls of the imaging methods and future prospects. This text should also help to understand image interpretation and the use of each of the methods and their combinations in various clinical situations as described in the final section of exemplary cases. We present the following article in accordance with the Narrative Review reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-22/rc).
Literature search and selection of sources
A bibliographical search was performed in PubMed using combinations of key words relating to “breast cancer”, “breast imaging”, “mammography”, “digital breast tomosynthesis”, “breast cancer screening”, “breast MRI”, “breast ultrasound”. The eligible criteria included studies in English language published between 2010 and 2021. We included studies referring to breast cancer imaging modalities and their use, breast cancer screening, treatment and survival related to imaging, and guidelines of special focus groups, medical societies and healthcare authorities. In particular, we focused on available meta-analyses and systematic reviews, large epidemiological studies, cohorts and case-control studies and randomized control trials. Reference lists from selected articles were also manually checked to identify additional relevant report.
Preventive versus diagnostic imaging
Mammography screening programmes have been running in many countries and detect on average 5 cancers per 1,000 screens (1). Attendance of mammographic screening has proven effective by randomized control trials in reducing the mortality of breast cancer by approximately 30% (2). Even in the era of modern therapy, detection of breast cancer in the early stages is the key to a better chance of survival (3). The greatest benefit achieved by screening mammography has been demonstrated for women between 50 and 69 years of age with up to a 40% reduction of mortality for women attending the screening programme (4). For the population between 40 and 49 years of age, the value of preventive mammography surveillance is still being discussed, but the evidence of the benefits for this age group has been increasing (5,6). The recommended screening interval is 2 years for the age category of 50–69 years and 1 year for women of 40–49 years of age, due to a higher mammographic density and greater aggressiveness of tumours in younger women (7).
Mammography screening programmes have been thoroughly scrutinized to evaluate potential adverse outcomes; mainly false positivity and overdiagnosis. The programme efficacy varies slightly in different countries, but in general the benefits outweigh the harms (8,9). The false positivity of mammographic screening is relatively low, reaching a maximum of 20% per 20 years of surveillance (10 screen rounds), and most of the findings are solved without any need for an interventional procedure; less than 1% of false positive findings require a core biopsy per screening round (4). Overdiagnosis (i.e., the rate of screen-diagnosed cancer which would otherwise go unnoticed during the patient’s lifetime), is estimated to additional 6.5% of cancers on average (ranging from 1% to 10%) (10).
Intensive preventive programmes in shorter time intervals (annual or even more frequent) are recommended for women with risk factors, especially a family history of breast/ovarian cancer and for genetic mutation carriers (11) with impact on improved survival (12,13). The protocols for women with an elevated or high risk of breast cancer involve multiple imaging modalities combining mammography with ultrasound and/or MRI which help detect more cancers in the earlier stages (14).
Diagnostic assessment is carried out on women of any age with clinical symptoms. These usually include a palpable lump in the breast or axilla, nipple discharge (especially when serous or bloody), skin changes or nipple or skin retraction. Clinically manifesting cancers typically comprise cancers in women of ages outside the screening period, women who do not attend preventive surveillance and interval cancers. Tumours manifesting clinically are usually larger and more advanced than tumours diagnosed in screening, with a higher risk of lymph node involvement, resulting in poorer prognosis (15).
Mammography
The basic imaging modality of the breast is mammography. This method uses low doses of ionizing radiation, therefore radiation exposure is minimal, ranging from 1.5 to 4 mGy, varying across countries and device manufacturers (16). Two views from each breast are obtained—one in cranio-caudal view, one in medio-lateral oblique view, which also enables evaluation of part of the axilla. Compression of the breast is necessary to reduce superposition of structures and decrease radiation dose (17). Additional views including magnification views, spot compression, rolled or extended views can be used to more clearly depict abnormalities.
Tumours are seen in mammography as mass lesions of higher density, with irregular or spiculated margins (Figure 1). Sometimes cancers can manifest as asymmetrical densities, distortions of breast parenchyma or smoothly contoured masses (which are otherwise more typical for benign processes such as cysts or fibroadenomas). The presence of microcalcifications, especially if these are clustered, follow ductal anatomy, are new or progress in time can also indicate malignancy. These typically represent ductal carcinoma in situ (DCIS) (18).
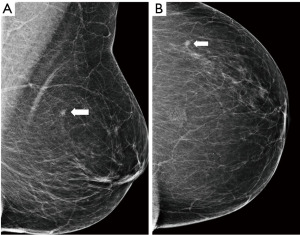
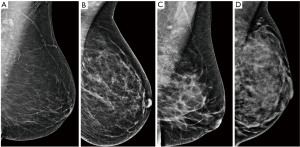
The performance of mammography is dependent on the breast density, which is determined by the proportion of glandular parenchyma and fat. The density is scored by the BI-RADS system from A (fatty) to D (dense) (Figure 2) and the sensitivity of mammography varies accordingly. In fatty breasts almost no cancer goes undetected, while in dense breasts the sensitivity can drop down to 50% (19). High breast density is an independent risk factor for breast cancer (20) and is also associated with higher proportion of interval cancers as smaller cancers can be masked by the dense parenchyma during screening (21). The density tends to change during life, decreasing with age. Mammography is therefore used and is more efficient in women over 40 years of age (22).
Technical innovations
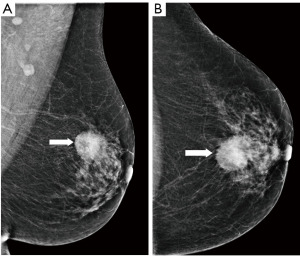
Digital breast tomosynthesis (DBT) is a novel approach in mammography that has the potential to overcome the limits of conventional mammography. It acquires several low-dose images of the breast, and reconstructs a synthetic 2D image with enhanced parenchymal distortion features and multiple slabs/slices of the breast, to enable exploration of 3D anatomy of the breast tissue (Figure 3). DBT detects approx. 15–30% more cancers, which would otherwise be hidden in the breast parenchyma in conventional mammography, and also helps to reduce the false positivity caused by superposition of normal structures mimicking pathology by 15–20% (23). Although very promising, DBT is still used mainly within clinical trials and its broader use as a screening method is still not routinely adopted. In comparison with mammography, DBT requires longer reading time, the radiation dose can be slightly higher and achievement of the main goal—reduction of interval cancers—has not yet been confidently demonstrated (24).
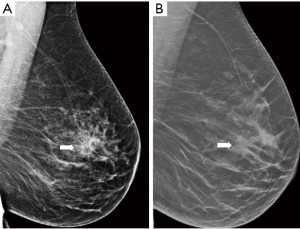
As a diagnostic tool DBT provides improved diagnostic accuracy compared with mammography and helps better localization of the lesions, distinguishing between benign and malignant features or detecting multifocality.
Staging with mammography
In staging, mammography is mainly important for evaluation of microcalcifications, as these may not be seen in other modalities and can represent a DCIS component.
Digital breast tomosynthesis can be useful for assessment of lesion size and identification of additional lesions in multifocal processes (25).
Breast ultrasound
Breast ultrasound has improved significantly during the last decades due to the advances in the technology and resolution of the devices. This method uses reflection of acoustic waves in the tissue and is a safe and well tolerated method for every patient. The main disadvantage is that a hand-held ultrasound is an operator dependent method, therefore the results of the examination may vary. Automated breast ultrasound systems (ABUS) might bring more reproducible and objective results (26).
Ultrasound should not be used as a standalone screening method (27) but is a valuable adjunct and diagnostic tool. In combination with mammography, ultrasound helps detect more cancers especially in the population of women with dense breasts where up to 4 additional cancers per 1,000 screened women can be found (28). Therefore, ultrasound can be recommended as a supplemental method to the mammography in women with breast density category D (very dense) and category C (heterogeneously dense) (29)and also in women with elevated risk (14). However, the data also consistently suggests that the addition of ultrasound brings increased false positivity and necessity for additional procedures or check-ups. Its routine role in the screening systems is therefore still being evaluated, as additional costs and the capacity of ultrasound centres must also be taken into account (30).
For evaluation of young, pregnant and breastfeeding patients with clinical symptoms breast ultrasound is used as the first (and usually sufficient) method. In this population, ultrasound reliably differentiates benign findings from those requiring a biopsy (31).
Breast ultrasound is also very helpful for evaluation of abnormities detected by mammography or MRI and navigation interventional methods such as biopsies and needle aspirations (32).
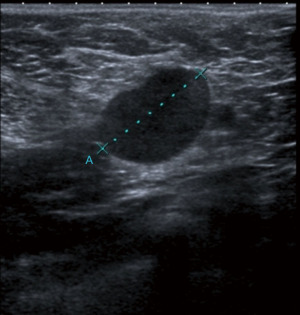
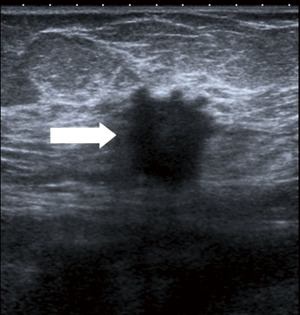
Breast cancer usually appears in ultrasound as hypoechoic (dark) mass with irregular margins, with vertical orientations and/or accompanied by posterior, acoustic shadowing. Some tumours can have an infiltrative pattern of growth appearing as non-circumscribed areas of decreased echogenicity (darker than normal parenchyma) (Figure 4). Ultrasound reliably differentiates between cystic and solid lesions.
Staging of breast cancer with ultrasound
Breast cancer frequently occurs as multiple lesions in one quadrant (multifocal) or multiple quadrants (multicentric) (33). In evaluation of patients with breast cancer ultrasound is a useful method for assessment of the extent of the disease and detection of additional lesions. Most additional lesions occur in the same quadrant, however detection of more distant additional lesions or even contralateral pathology is not rare and may alter the planning of the treatment.
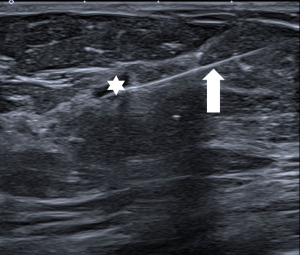
Ultrasound of the axilla (axillary ultrasound) is mandatory for staging of the disease. Various features of lymph nodes are considered suspicious of metastatic involvement: cortical thickening of more than 3mm, irregular cortex width, displacement or absence of the fatty hilum or round shape of the lymph node (Figure 5). Ultrasound is also the method used to navigate the fine needle aspiration biopsy (FNA/FNAB) to confirm the status of the lymph node (34), with high sensitivity (79.6%), specificity (98.3%) and PPV 97.1% for identification of axillary involvement. With decreasing radicality of axillary surgery, the main advantage of axillary ultrasound is its ability to reliably identify or exclude a major axillary tumour burden (35).
Breast magnetic resonance imaging (MRI)
Breast MRI is an established valuable method that helps detect lesions that are not visible for other modalities. The sensitivity of dynamic contrast-enhanced MRI (DCE-MRI), 93%, is very high for every type of breast (including dense breasts) with relatively good specificity of 71% (36). The examination requires the application of a gadolinium-based contrast agent intravenously, and there are several contraindications as this method uses a high-intensity magnetic field.
The clinical indications for the use of breast MRI include high-risk screening, staging of breast cancer, evaluation of the effect of neoadjuvant chemotherapy, detection of occult breast cancer, evaluation of implants, evaluation of nipple discharge and assessment of equivocal lesions in conventional imaging methods (37).
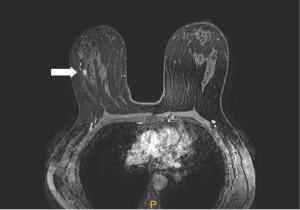
In gene mutation carriers and in women with a high risk (>20%) of breast cancer, MRI is superior to all other breast imaging methods for the early detection of cancer and is recommended for surveillance of this population (38,39) (Figure 6).
MRI also detects more early cancers than mammography in women with a family history of breast cancer but without proven genetic mutation (40) and in women with extra dense breast tissue. In the DENSE trial (41) with MRI used as a supplemental method to mammography, the ultimate goal of significantly reducing interval cancers (2.5/1,000 with MRI versus 5/1,000 for mammography only) was reached. Interestingly, MRI also achieves a high cancer detection rate in the average risk population of variable densities (42). The additional detection rate of 15.5 per 1,000 is much higher than that of any other imaging methods. Most of these studies however also suggest a higher proportion of false positive results than with mammography, which need further evaluation including interventions under MRI guidance.
The availability, price and duration of the examination and the interpretation time have always been raised as issues which have prevented wider use. Abbreviated protocol, shortening both image acquisition and study evaluation time while maintaining the same diagnostic accuracy, could help solve these issues and make MRI available to more patients (43) (Figure 7).
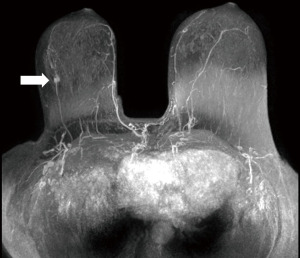
In MRI, cancer typically appears as a mass with irregular shape, lobulated or spiculated margins and inhomogeneous structure, or as non-mass-like areas with ductal or segmental distribution, both with marked and/or early enhancement in dynamic post-contrast sequence which decreases in later phases. The dynamic contrast-enhanced sequence is the most important sequence for detection of malignant lesions, the additional MRI sequences [T2-weighted sequences, diffusion-weighted imaging (DWI), spectroscopy] can further characterize the pathology and help differentiate malignant lesions from benign processes. The combined use of multiple parameters of MRI further increases specificity of the method to up to 75–89% (44).
Staging with MRI
In staging of a biopsy-proven breast cancer, MRI is often used for the assessment of the extent of the disease and detection of additional lesions in the same or in the contralateral breast, which potentially influence the patient´s subsequent management. Due to its high sensitivity MRI is superior to mammography and ultrasound in identification of a DCIS component or multifocality. MRI is frequently used in lobular histology of the cancer, in patients with dense breasts, younger in age, in case of discrepancy of the lesion size in mammography, ultrasound or clinical findings and in uncertainty of the extent or suspected multifocal/multicentric disease detected with mammography and ultrasound.
While the value of MRI has been questioned in the past as increased mastectomy rate was observed and the benefits affecting reexcision and survival rates had not been demonstrated previously (45), recent studies support the use of MRI in various scenarios with a proven reduction in the breast reoperation rate from 15% to 5% (46).
Biopsy techniques
Each lesion found in imaging where malignancy cannot be ruled out must be biopsied. Various procedures under imaging guidance are available (47). The FNA/FNAB obtains clusters of cells, enables differentiation of malignant from benign findings and evaluates metastatic involvement of axillary lymph nodes. The core biopsy (12–16G with standard 14G) retrieves pieces of compact tissue, thus enabling the additional assessment of the biological and prognostic markers of the tumour, which possibly have an impact on the treatment choices. The vacuum-assisted biopsy (VAB) uses larger-gauge needles, providing samples with a larger amount of tissue.
Imaging is used for precise navigation of the procedure. The modality where the lesion is most visible is always used for guidance. The easiest way to target a biopsy needle is under ultrasound guidance, which enables real-time navigation (Figure 8). The VAB is frequently navigated by mammography for a biopsy of microcalcifications that are not visible by ultrasound. The VAB can also be used under MRI guidance for lesions visible only by MRI.
The multimodality approach for staging and management
No modality stands alone in the evaluation and staging of breast cancer. Clinical information about the patient, clinical findings, imaging studies and patient’s preferences must all be combined in planning strategy.
In many patients the combination of mammography and ultrasound provide sufficient information about the breast and the axilla for planning of the strategy. In some cases, MRI is necessary to help detect additional lesions or to evaluate the extent of the disease (see examples of clinical scenarios below). Each lesion that is found in additional imaging and which would alter the treatment plan must undergo further evaluation and a biopsy. Lesions detected by MRI must be evaluated with special caution, as a potentially false positive finding, due to the high sensitivity of this method, may result in unnecessarily radical surgery.
Clinical practice has shown that patients benefit from therapeutic management based on a multidisciplinary approach, which involves multiple specialties and a patient´s perspective. The multidisciplinary team (MDT) includes the radiologist, pathologist, surgeon, oncologist, radiation oncologist and the breast nurse/psychologist. Regular MDT meetings where each breast cancer case is discussed help review all the information from imaging, relevant clinical patient data and patients’ preferences and help to plan how to proceed. The complexity of the combined multidisciplinary approach, which does not bring merely a summary of findings, translates into an 18% increase in the survival rate, as shown by a United Kingdom study (48).
Preoperative marking
Preoperative or pretreatment marking of non-palpable tumour lesions, and possibly also axillary involvement, are vital for transferring information from imaging to surgery. The method of marking is dependent on the centre’s preferences and is discussed in a multidisciplinary team meeting. A variety of localization wires, clips visible by ultrasound, detectable by magnetic or scintillation probe are available, supplemented by skin or carbon markings. For larger lesions, marking with multiple wires/clips/marks (“bracketing”) is necessary to ensure proper localization and delineation of the extent of the pathological finding. Protocols and standard practices with close cooperation of the radiologist and the surgeon are used to ensure the best outcomes. Each lesion is marked under guidance of the method where both the localization and whole extent is most visible. Ultrasound is the easiest method for any intervention, however in cases of microcalcifications which are not visible by ultrasound, mammography (stereotactic) guidance might be necessary. For MRI-only detected lesions, biopsy and localization might be more challenging, but it is necessary in order to ensure an optimal outcome (49).
If neoadjuvant chemotherapy is planned for the patient, with a subsequent scheduled attempt at breast conserving surgery, early marking is mandatory for all the lesions, as these may disappear during the treatment. The same applies for the affected lymph nodes if a targeted lymph node dissection is to be attempted (50).
Examples of clinical scenarios
- Screening, a patient of 50 years in age, no clinical finding, mammography with fat predominance (density A), new dense nodule with spiculated margins and microcalcifications is present on the left side. The lesion is biopsied under ultrasound guidance with result of low grade carcinoma, lymph nodes negative. No further assessment necessary, the patient is scheduled for breast conserving surgery with a sentinel node biopsy (Figure 9).
- Screening, an asymptomatic woman of 65 years old, in mammography an architectural distortion is detected in the lateral part of the left breast. The ultrasound findings are subtle with suggested areas of decreased echogenicity. The biopsy under ultrasound guidance reveals DCIS grade 2. The extent of the disease however is not certain. MRI is indicated. MRI shows an extensive process in the lateral part of the left breast resulting in the need of mastectomy (Figure 10).
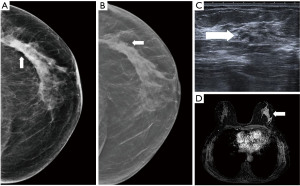 Figure 10 Mammography of the left breast in cranio-caudal view (A) with architectural distortion (arrow). The distortion is more visible in DBT (B) (arrow). Ultrasound (C) reveals subtle finding of irregular area of decreased echogenicity (arrow). MRI (D) shows extensive area of non-mass-like enhancement in the whole lateral part of the breast (arrow). DBT, digital breast tomosynthesis; MRI, magnetic resonance imaging.
Figure 10 Mammography of the left breast in cranio-caudal view (A) with architectural distortion (arrow). The distortion is more visible in DBT (B) (arrow). Ultrasound (C) reveals subtle finding of irregular area of decreased echogenicity (arrow). MRI (D) shows extensive area of non-mass-like enhancement in the whole lateral part of the breast (arrow). DBT, digital breast tomosynthesis; MRI, magnetic resonance imaging. - Diagnostic assessment, a patient of 45 years of age with a palpable lump on the right side for 2 months. In mammography with higher proportion of fibroglandular tissue (category C) several areas of increased density with irregular margins and architectural distortions are visible. Ultrasound confirms more than one lesion. MRI demonstrates a large area of enhancement up to 7 cm (Figure 11).
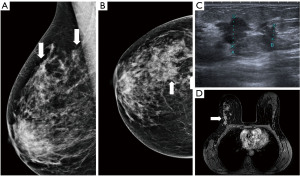 Figure 11 Mammography of the right breast in medio-lateral-oblique (A) and cranio-caudal view (B) with multiple densities with irregular margins and architectural distortions (arrows). Ultrasound (C) shows more than two hypoechoic lesions of suspicious features. In MRI (D) an extensive area of tumour involvement is revealed. MRI, magnetic resonance imaging.
Figure 11 Mammography of the right breast in medio-lateral-oblique (A) and cranio-caudal view (B) with multiple densities with irregular margins and architectural distortions (arrows). Ultrasound (C) shows more than two hypoechoic lesions of suspicious features. In MRI (D) an extensive area of tumour involvement is revealed. MRI, magnetic resonance imaging. - A patient of 50 years of age evaluated for enlarged lymph nodes in the axilla. Mammography and ultrasound show enlarged pathological lymph nodes in the axilla, otherwise no pathological finding in the breast on initial evaluation despite the low mammographic density. The largest lymph node is biopsied proving metastatic invasive carcinoma NST of breast origin. MRI is indicated to search for an occult lesion in the breast. MRI shows the enlarged lymph nodes and a small lesion in the right breast in upper outer quadrant. A second-look, targeted ultrasound with the knowledge of the location of the lesion is performed to reveal a small suspicious lesion, which is subsequently verified as the primary tumour in the breast (Figure 12).
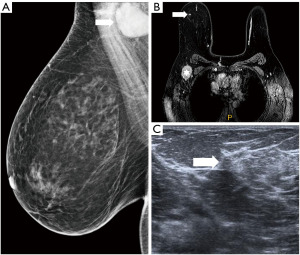 Figure 12 Enlarged lymph node in the right axilla is seen in mammography (A). In MRI (B) enlarged lymph nodes (star) are confirmed and a small mass with early intense enhancement in the upper outer quadrant (arrow). In ultrasound (C) the lesion is very subtle (arrow). MRI, magnetic resonance imaging.
Figure 12 Enlarged lymph node in the right axilla is seen in mammography (A). In MRI (B) enlarged lymph nodes (star) are confirmed and a small mass with early intense enhancement in the upper outer quadrant (arrow). In ultrasound (C) the lesion is very subtle (arrow). MRI, magnetic resonance imaging. - Preoperative marking. Microcalcification with ductal distribution biopsied by vacuum—assisted biopsy under mammography guidance as DCIS grade 2; the extent of the calcifications is approximately 30 mm. Marking by two wires is performed to delineate the extent of the disease. A specimen mammography of the resected tissue shows both wires with microcalcifications between them that do not reach the margins (Figure 13).
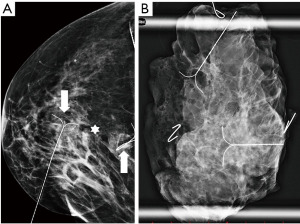 Figure 13 In mammography (A) microcalcifications are visible in the centre of the breast (star). The extent is delineated by two wires placed to the ventral and dorsal edge of the microcalcifications (arrows). A specimen mammography (B) confirms both wires and microcalcifications present in the removed tissue, the microcalcifications do not reach the margins of the specimen.
Figure 13 In mammography (A) microcalcifications are visible in the centre of the breast (star). The extent is delineated by two wires placed to the ventral and dorsal edge of the microcalcifications (arrows). A specimen mammography (B) confirms both wires and microcalcifications present in the removed tissue, the microcalcifications do not reach the margins of the specimen.
Summary
Breast imaging is complex and still evolving. Preventive programmes are seeking more effective ways of detecting more cancers in the earlier stages. For staging purposes, a multimodality approach using a combination of multiple imaging methods is necessary for proper planning of the patient’s subsequent management. Preoperative marking ensures transfer of the information from imaging to surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard and Jørn Bo Thomsen) for the series “Breast Reconstruction - The True Multidisciplinary Approach” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-22/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-22/coif). The series “Breast Reconstruction - The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lehman CD, Arao RF, Sprague BL, et al. National Performance Benchmarks for Modern Screening Digital Mammography: Update from the Breast Cancer Surveillance Consortium. Radiology 2017;283:49-58. [Crossref] [PubMed]
- Dibden A, Offman J, Duffy SW, et al. Worldwide Review and Meta-Analysis of Cohort Studies Measuring the Effect of Mammography Screening Programmes on Incidence-Based Breast Cancer Mortality. Cancers (Basel) 2020;12:976. [Crossref] [PubMed]
- Saadatmand S, Bretveld R, Siesling S, et al. Influence of tumour stage at breast cancer detection on survival in modern times: population based study in 173,797 patients. BMJ 2015;351:h4901. [Crossref] [PubMed]
- Lauby-Secretan B, Scoccianti C, Loomis DInternational Agency for Research on Cancer Handbook Working Group, et al. Breast-cancer screening--viewpoint of the IARC Working Group. N Engl J Med 2015;372:2353-8. [Crossref] [PubMed]
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol 2020;21:1165-72. [Crossref] [PubMed]
- Ray KM, Joe BN, Freimanis RI, et al. Screening Mammography in Women 40-49 Years Old: Current Evidence. AJR Am J Roentgenol 2018;210:264-70. [Crossref] [PubMed]
- Sardanelli F, Aase HS, Álvarez M, et al. Position paper on screening for breast cancer by the European Society of Breast Imaging (EUSOBI) and 30 national breast radiology bodies from Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Israel, Lithuania, Moldova, The Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Sweden, Switzerland and Turkey. Eur Radiol 2017;27:2737-43. [Crossref] [PubMed]
- Hofvind S, Ponti A, Patnick JEUNICE Project and Euroscreen Working Groups, et al. False-positive results in mammographic screening for breast cancer in Europe: a literature review and survey of service screening programmes. J Med Screen 2012;19:57-66. [Crossref] [PubMed]
- Løberg M, Lousdal ML, Bretthauer M, et al. Benefits and harms of mammography screening. Breast Cancer Res 2015;17:63. [Crossref] [PubMed]
- Paci EEUROSCREEN Working Group. Summary of the evidence of breast cancer service screening outcomes in Europe and first estimate of the benefit and harm balance sheet. J Med Screen 2012;19:5-13. [Crossref] [PubMed]
- Paluch-Shimon S, Cardoso F, Sessa CESMO Guidelines Committee, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol 2016;27:v103-v110. [Crossref] [PubMed]
- Bae MS, Sung JS, Bernard-Davila B, et al. Survival Outcomes of Screening with Breast MRI in Women at Elevated Risk of Breast Cancer. J Breast Imaging 2020;2:29-35. [Crossref] [PubMed]
- Evans DG, Kesavan N, Lim Y, et al. MARIBS Group. MRI breast screening in high-risk women: cancer detection and survival analysis. Breast Cancer Res Treat 2014;145:663-72. Corrected in Breast Cancer Res Treat 2014;147:689. [Crossref] [PubMed]
- Berg WA, Zhang Z, Lehrer DACRIN 6666 Investigators, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012;307:1394-404. [Crossref] [PubMed]
- Domingo L, Blanch J, Servitja S, et al. Aggressiveness features and outcomes of true interval cancers: comparison between screen-detected and symptom-detected cancers. Eur J Cancer Prev 2013;22:21-8. [Crossref] [PubMed]
- Suleiman ME, Brennan PC, McEntee MF. Diagnostic reference levels in digital mammography: a systematic review. Radiat Prot Dosimetry 2015;167:608-19. [Crossref] [PubMed]
- Dance DR, Sechopoulos I. Dosimetry in x-ray-based breast imaging. Phys Med Biol 2016;61:R271-R304. [Crossref] [PubMed]
- Rauch GM, Hobbs BP, Kuerer HM, et al. Microcalcifications in 1657 Patients with Pure Ductal Carcinoma in Situ of the Breast: Correlation with Clinical, Histopathologic, Biologic Features, and Local Recurrence. Ann Surg Oncol 2016;23:482-9. [Crossref] [PubMed]
- Weigel S, Heindel W, Heidrich J, et al. Digital mammography screening: sensitivity of the programme dependent on breast density. Eur Radiol 2017;27:2744-51. [Crossref] [PubMed]
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:1159-69. [Crossref] [PubMed]
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and Clinical Breast Imaging Reporting and Data System Density Measures Predict Risk for Screen-Detected and Interval Cancers: A Case-Control Study. Ann Intern Med 2018;168:757-65. [Crossref] [PubMed]
- Checka CM, Chun JE, Schnabel FR, et al. The relationship of mammographic density and age: implications for breast cancer screening. AJR Am J Roentgenol 2012;198:W292-5. [Crossref] [PubMed]
- Gilbert FJ, Tucker L, Young KC. Digital breast tomosynthesis (DBT): a review of the evidence for use as a screening tool. Clin Radiol 2016;71:141-50. [Crossref] [PubMed]
- Giampietro RR, Cabral MVG, Lima SAM, et al. Dos Santos Nunes-Nogueira V. Accuracy and Effectiveness of Mammography versus Mammography and Tomosynthesis for Population-Based Breast Cancer Screening: A Systematic Review and Meta-Analysis. Sci Rep 2020;10:7991. [Crossref] [PubMed]
- Michell MJ, Batohi B. Role of tomosynthesis in breast imaging going forward. Clin Radiol 2018;73:358-71. [Crossref] [PubMed]
- Zanotel M, Bednarova I, Londero V, et al. Automated breast ultrasound: basic principles and emerging clinical applications. Radiol Med 2018;123:1-12. [Crossref] [PubMed]
- Evans A, Trimboli RM, Athanasiou A, et al. Breast ultrasound: recommendations for information to women and referring physicians by the European Society of Breast Imaging. Insights Imaging 2018;9:449-61. [Crossref] [PubMed]
- Rebolj M, Assi V, Brentnall A, et al. Addition of ultrasound to mammography in the case of dense breast tissue: systematic review and meta-analysis. Br J Cancer 2018;118:1559-70. [Crossref] [PubMed]
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental Screening for Breast Cancer in Women With Dense Breasts: A Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med 2016;164:268-78. [Crossref] [PubMed]
- Lee JM, Arao RF, Sprague BL, et al. Performance of Screening Ultrasonography as an Adjunct to Screening Mammography in Women Across the Spectrum of Breast Cancer Risk. JAMA Intern Med 2019;179:658-67. [Crossref] [PubMed]
- Lehman CD, Lee CI, Loving VA, et al. Accuracy and value of breast ultrasound for primary imaging evaluation of symptomatic women 30-39 years of age. AJR Am J Roentgenol 2012;199:1169-77. [Crossref] [PubMed]
- Hooley RJ, Scoutt LM, Philpotts LE. Breast ultrasonography: state of the art. Radiology 2013;268:642-59. [Crossref] [PubMed]
- Tot T, Gere M, Hofmeyer S, et al. The subgross morphology of breast carcinomas: a single-institution series of 2033 consecutive cases documented in large-format histology slides. Virchows Arch 2020;476:373-81. [Crossref] [PubMed]
- Mainiero MB, Cinelli CM, Koelliker SL, et al. Axillary ultrasound and fine-needle aspiration in the preoperative evaluation of the breast cancer patient: an algorithm based on tumor size and lymph node appearance. AJR Am J Roentgenol 2010;195:1261-7. [Crossref] [PubMed]
- Ahmed M, Jozsa F, Baker R, et al. Meta-analysis of tumour burden in pre-operative axillary ultrasound positive and negative breast cancer patients. Breast Cancer Res Treat 2017;166:329-36. [Crossref] [PubMed]
- Zhang L, Tang M, Min Z, et al. Accuracy of combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging for breast cancer detection: a meta-analysis. Acta Radiol 2016;57:651-60. [Crossref] [PubMed]
- Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 2010;46:1296-316. [Crossref] [PubMed]
- Riedl CC, Luft N, Bernhart C, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol 2015;33:1128-35. [Crossref] [PubMed]
- Sardanelli F, Podo F, Santoro F, et al. High Breast Cancer Risk Italian 1 (HIBCRIT-1) Study. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the high breast cancer risk italian 1 study): final results. Invest Radiol 2011;46:94-105. [Crossref] [PubMed]
- Saadatmand S, Geuzinge HA, Rutgers EJT, et al. FaMRIsc study group. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): a multicentre, randomised, controlled trial. Lancet Oncol 2019;20:1136-47. [Crossref] [PubMed]
- Bakker MF, de Lange SV, Pijnappel RMDENSE Trial Study Group, et al. Supplemental MRI Screening for Women with Extremely Dense Breast Tissue. N Engl J Med 2019;381:2091-102. [Crossref] [PubMed]
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental Breast MR Imaging Screening of Women with Average Risk of Breast Cancer. Radiology 2017;283:361-70. [Crossref] [PubMed]
- Leithner D, Moy L, Morris EA, et al. Abbreviated MRI of the Breast: Does It Provide Value? J Magn Reson Imaging 2019;49:e85-e100. [Crossref] [PubMed]
- Marino MA, Helbich T, Baltzer P, Pinker-Domenig K. Multiparametric MRI of the breast: A review. J Magn Reson Imaging 2018;47:301-15. [Crossref] [PubMed]
- Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg 2013;257:249-55. [Crossref] [PubMed]
- Gonzalez V, Sandelin K, Karlsson A, et al. Preoperative MRI of the breast (POMB) influences primary treatment in breast cancer: a prospective, randomized, multicenter study. World J Surg 2014;38:1685-93. [Crossref] [PubMed]
- Bick U, Trimboli RM, Athanasiou A, et al. Image-guided breast biopsy and localisation: recommendations for information to women and referring physicians by the European Society of Breast Imaging. Insights Imaging 2020;11:12. [Crossref] [PubMed]
- Kesson EM, Allardice GM, George WD, et al. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ 2012;344:e2718. [Crossref] [PubMed]
- Kuhl CK, Strobel K, Bieling H, et al. Impact of Preoperative Breast MR Imaging and MR-guided Surgery on Diagnosis and Surgical Outcome of Women with Invasive Breast Cancer with and without DCIS Component. Radiology 2017;284:645-55. [Crossref] [PubMed]
- Dubsky P, Pinker K, Cardoso F, et al. Breast conservation and axillary management after primary systemic therapy in patients with early-stage breast cancer: the Lucerne toolbox. Lancet Oncol 2021;22:e18-e28. [Crossref] [PubMed]
Cite this article as: Steyerova P, Burgetova A. Current imaging techniques and impact on diagnosis and survival —a narrative review. Ann Breast Surg 2022;6:25.