Narrative review of breast reconstruction with a latissimus dorsi flap—is there a price to pay?
IntroductionOther Section
- Introduction
- Surgical technique
- Range of motion (ROM)
- Shoulder strength
- Patient-reported shoulder function
- Other aspects
- Conclusions
- Acknowledgments
- Footnote
- References
The predecessor of the modern musculocutaneous latissimus dorsi flap (m. latissimus dorsi flap) was initially described more than 100 years ago by Italian surgeon Ignicio Tansini as an option for coverage of large defects after breast surgery (1). Since the 1970s when the flap technique was modernized and adapted for breast reconstruction, it has become increasingly popular and today remains a workhorse in reconstructive plastic surgery (2).
Breast cancer incidence has been rising for decades and today more than 1 out of 10 women are diagnosed with breast cancer. Fortunately, the increased knowledge about diagnosis and adjuvant therapies to surgical treatment have left the 5-year survival rate at more than 85% (3), thus creating an increased need for reconstructive procedures in order to help alleviate the physiological and psychological trauma related to a cancer diagnosis (4).
Autologous breast reconstruction is the preferred option of many surgeons for patients in need of secondary reconstructive procedures after radiation therapy (5). Radiotherapy can result in hard, fibrotic tissue in the area of the removed breast, which makes implant-based reconstructions difficult and necessitates the addition of healthy tissue (6). The latissimus dorsi (LD) flap supplies a natural skin island and underlying soft tissue to the damaged area, and provides a natural appearance and texture of the reconstructed breast.
The impact on shoulder function following LD-flap breast reconstruction has been discussed for years, and numerous studies have examined the effect of LD harvest through the past five decades (7-17). Despite different authors presenting some degree of measurable loss of shoulder strength following LD transfer, the subjective functional outcome and effect on patient’s ability to perform activities of daily living (ADL) remains unresolved (8-12,17-20).
Clarity regarding factors other than donor-site morbidity, such as the length of postoperative hospitalization, complication-rates, aesthetic outcome, and the expected need for corrective procedures is needed in order to properly evaluate the benefits and drawbacks of breast reconstruction with an LD-flap.
The following narrative review aims at presenting an overview of the impact on shoulder function following breast reconstruction with a latissimus dorsi flap, with respect to measurable changes in shoulder motion and strength as well as ability to perform activities of daily living. We present the following article in accordance with the Narrative Review reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-30/rc).
Surgical techniqueOther Section
- Introduction
- Surgical technique
- Range of motion (ROM)
- Shoulder strength
- Patient-reported shoulder function
- Other aspects
- Conclusions
- Acknowledgments
- Footnote
- References
The patients are typically placed in the lateral position, with the arm elevated and pointed forward to allow dissection in the axillary area. The surgery is usually initiated with dissection of the thoracodorsal vessels, that constitutes the vascular supply. This should be done with caution as previous lymph node dissection or radiation therapy to the area may have left the thoracodorsal vessels surrounded by fibrotic scar tissue. In rare cases, the thoracodorsal vessels might have been damaged during lymph node dissection, which would necessitate an alternative reconstructive strategy. Some surgeons advocate the pre-operative use of color-doppler ultrasonography, which may be a very useful tool to detect vascular anomalies and plan an alternative approach. The vascular pedicle is dissected from its insertion into the latissimus dorsi muscle and toward the axilla until the desired length of the pedicle is achieved—often about 8–10 cm, but can reach up to 15 cm.
The nerve is the identified and, may be ligated, depending on the surgeon’s preferences, in an attempt to avoid jumping breast syndrome (21). The flap is then dissected, with respect to the desired size of skin island, in its entity from its origin at the lower back and the dissection continues towards its most inferior part at the iliac crest, from where the last part of the dissection is performed in direction of the axilla. Some surgeons prefer to initiate the dissection from the lumbar origin of the muscle and proceed towards the axilla, which may be a time-sparing option, if the thoracodorsal vessels have been identified as functional preoperatively. The humeral insertion may then be detached, and the flap is transposed through the axilla, to its new position at the chest.
A number of variations to this technique has been described throughout the years and the alternatives include the extended myocutaneous LD flap where a portion of the lumbar fat is included in the flap, in order to provide sufficient volume for complete breast reconstruction (22). On the other hand is the muscle-sparing LD flap where a strip of muscle is kept to protect the vessels and constitute a pedicle based on the descending branch of the thoracodorsal artery, while the remaining part of the muscle is left functional at its original place (23). The perforator-based alternative, the thoracodorsal artery perforator (TDAP) flap, is this donor-area’s analogue to the deep inferior epigastric perforator (DIEP) flap, that consists of skin and underlying fascia and is supplied by perforators from the thoracodorsal artery. It is usually “propelled” to the its new position at the site of breast reconstruction (24).
Range of motion (ROM)Other Section
- Introduction
- Surgical technique
- Range of motion (ROM)
- Shoulder strength
- Patient-reported shoulder function
- Other aspects
- Conclusions
- Acknowledgments
- Footnote
- References
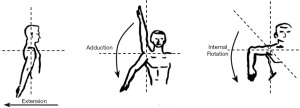
Change in ROM is a popular and easy-to-asses method for determining change of shoulder function following LD breast reconstruction. It is typically measured using a goniometer (25), but different, digital, assessment methods have emerged in recent years, which provides an option that does not necessitate any equipment in excess of a smartphone and can be performed at any place by the physician (26). ROM should ideally be performed pre- and postoperatively, and with a follow-up that respects the physiological changes and maturing of scar-tissue after surgery. The LD muscle primarily contributes to the shoulder motions extension, adduction, and internal rotation (Figure 1).
Several studies have examined the changes in ROM after LD transfer, although only three studies report the specific changes following breast reconstruction with the pedicled LD flap (Table 1). Russell et al. investigated 24 patients of which 7 had breast reconstruction performed (7). The patients with breast reconstruction had a 13.5% decrease in internal rotation and a decrease of 5.6% for extension across the cohort. No change was detected for abduction. Glassey et al. investigated 22 patients with a 12-month follow-up and found an increase of shoulder extension of 4.4 degrees and an increase of 8.0 degrees for internal rotation (10). Sowa et al. examined 18 patients and found a non-significant increase in extension of 3.1 degrees and 6.4 degrees at 12 and 36 months respectively. Internal rotation was limited by 2.0 degrees at 12 months but had improved by 0.8 degrees at 36 months follow-up but was not statistically significant (16). de Oliveira et al. found no significant change in ROM 1 year after immediate breast reconstruction with an LD flap, but investigated shoulder flexion and abduction, motions to which the LD muscle is not usually considered to contribute (13). Other studies have reported some changes in shoulder ROM but have not reported quantified results as Garusi et al. (14) report that 96% of their cohort of 86 patients had recovered shoulder ROM of 80–100% for extension and 94% had recovered 80–100% of internal rotation at the end of follow-up which ranged from 1–14 years. Saint-Cyr et al. investigated ROM between the operated and non-operated side in 20 patients and found no difference between the sides for any motions of the shoulder (11). Rindom et al. performed a randomized trial comparing LD to TAP flap breast reconstruction and found a decrease in Constant shoulder score of three points for the LD group at 12-month follow-up (17). The Constant score is system for assessment of shoulder function, that investigates ROM (0–40 points), pain (0–15 points), strength (0–25 points) and ability to perform activities of daily living (0–20 points) and scores the function from 0–100, with a score of 100 meaning no shoulder impairment at all.
Table 1
| Study | Number | Follow-up | Measurement | Change | P |
|---|---|---|---|---|---|
| Glassey et al. (in 2008) | 22 | 12 months | Adduction | 0 | NA |
| Extension | +4.4° | NA | |||
| Internal rotation | +8.0° | NA | |||
| Sowa et al. (in 2017) | 18 | 12 months | Extension | +3.1° | ns |
| 36 months | Extension | +6.4° | ns | ||
| 12 months | Internal rotation | −2.0° | ns | ||
| 36 months | Internal rotation | +0.9° | ns | ||
| Russel et al. (in 1986) | 23 | 16 months | Adduction | 0 | ns |
| – | Extension | 5.6% | ns | ||
| 7* | Internal rotation | 13.5% | ns |
*, specified for breast-reconstruction patients. ROM, range of motion; LD, latissimus dorsi; NA, not applicable; ns, no statistical significance.
The vast heterogeneity of reporting measures, study design and follow-up time makes it difficult to compare the effect of LD breast reconstruction on shoulder ROM, and whilst some report a decrease and others an increase, a definite conclusion remain unclear.
Shoulder strengthOther Section
- Introduction
- Surgical technique
- Range of motion (ROM)
- Shoulder strength
- Patient-reported shoulder function
- Other aspects
- Conclusions
- Acknowledgments
- Footnote
- References
Shoulder strength can be measured in a number of different ways but the gold standard for muscle testing is isokinetic dynamometry (27). The major drawback of the isokinetic dynamometer is its large size and immobility, but handheld devices for isometric dynamometry has shown good results regarding reproducibility and is often the preferred choice in studies of breast reconstruction patients. Measurements have also been performed using a spring balance or manual muscle testing (7,10), but these do not provide the same accuracy as the dynamometers. An overview of studies examining muscle-strength is shown in Table 2.
Table 2
| Study | n | Follow-up | Motion | Measurement | Change | P |
|---|---|---|---|---|---|---|
| Rindom et al. (in 2019) | 18 | 12 months | NA | Isometric | −18% | NA |
| Sowa et al. (in 2017) | 20 | 36 months | Adduction | Isometric | −36% | <0.05 |
| 36 months | Extension | −7% | ns | |||
| 36 months | Internal rotation | −14% | <0.05 | |||
| Van Huizum et al. (in 2016) | 12 | 3.5 years | Adduction | Isometric | 16.2% | <0.05 |
| Extension | −22.4% | <0.05 | ||||
| Internal rotation | −14.4% | <0.05 | ||||
| Forthomme et al. (in 2010) | 20 | 6 months | Adduction | Isokinetic | −31% | <0.05 |
| Internal rotation | −19% | <0.05 | ||||
| Glassey et al. (in 2008) | 22 | 12 months | Adduction | Spring balance | −0.3 kg | NA |
| Extension | −0.06 kg | NA | ||||
| Fraulin et al. (in 1995) | 13 | 4.4 years | Adduction | Isokinetic | −39% | <0.05 |
| Extension | −32% | <0.05 | ||||
| Internal rotation | −19% | ns | ||||
| Russel et al. (in 1986) | 23 | 16 months | Latissimus function | Manual | −18% | NA |
*, specified for breast-reconstruction patients. LD, latissimus dorsi; NA, not applicable; ns, no statistical significance.
Rindom et al. found a decrease in Constant score for shoulder strength of 2.2 points after measurements with a dynamometer, but evaluated against patients undergoing TAP flap reconstruction, and found no significant difference between the groups (17).
Sowa et al. used isometric testing of shoulder strength and found a significant decrease in adduction strength of 36% at 3-year follow-up and a significant decrease of 14% for internal rotation. Extension strength was decreased by 7% but insignificantly (16).
In 2016, van Huizum and colleagues performed a study of 12 women who had undergone LD breast reconstruction at an average of 3.5 years prior to the study (15). They investigated the loss of synergistic muscle strength and controlled to the contralateral arm. They found a significant decrease of shoulder strength for extension, adduction, and internal rotation of on the operated side compared to the non-operated and reported 19% higher scores for overall torque of the motions performed by the latissimus muscle, on the non-operated side. Forthomme et al. performed a study measuring shoulder strength with an isokinetic dynamometer in 20 women undergoing LD breast reconstruction with a follow-up of 6 months (12). They found a significant reduction of peak torque for internal rotation (19%) and adduction (31%) at the end of follow-up. The results were compared to the non-operated site, and the significant reductions were only present on the operated side. Fraulin et al. examined 13 women using isokinetic testing (9). Mean time from reconstruction was 4.4 years and strength were measured between the operated and the non-operated side. They found that shoulder strength was significantly reduced by 32% for extension and 39% for adduction. An insignificant decrease of 19% for internal rotation was also recorded. They also included isotonic functional strength tests using a Baltimore therapeutic equipment (BTE), that simulates activities such as ladder climbing, painting and ability to push up from a chair. The ability to utilize the shoulder for the mentioned activities was significantly reduced in breast reconstruction patients, while simulation of skiing was unaffected.
Other authors have utilized less reproducible methods for testing shoulder strength. Glassey and colleagues found a decrease in shoulder strength for extension of 0.06 kg and a reduction in adduction of 0.3 kg at 12-month follow up, but measured strength using a spring balance, and did not provide any statistical considerations alongside the results (10). Russell et al. used manual muscle testing and found that the operated side was statistically weaker in all patients. The average weakening when they specifically tested for strength of the latissimus dorsi muscle was 18%, which surprised they recorded some degree of strength “even though the muscle was gone” (7).
The results of the studies clearly show that some degree of measurable shoulder weakening should be expected following LD breast reconstruction, although it also appears that the agonistic muscles of the shoulder to some extent compensate for the loss as there seem to be an increase in strength from the earliest measurements (1–3 months) to 12-month follow-up.
Patient-reported shoulder functionOther Section
- Introduction
- Surgical technique
- Range of motion (ROM)
- Shoulder strength
- Patient-reported shoulder function
- Other aspects
- Conclusions
- Acknowledgments
- Footnote
- References
One issue is the determination of measurable shoulder weakening after LD breast reconstruction, another is whether this loss actually affects the patient’s everyday life and ability to perform daily activities. A wide variety of assessment options for patient-reported outcome measures (PROMs) is available when examining the functionality of the shoulder function. Most often has questionnaires been used and the preferred har traditionally been the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire, although different questionnaires and interviews have been individually designed in several different studies. Furthermore, the Constant score includes a section of the patients’ ability to perform ADL.
The studies by Forthomme et al. and Rindom et al. found a decrease in Constant score for ADL of 40% (6 months post-op) and 8% respectively (12 months post-op) (12,17) and the change in the latter study was significantly larger than for patients undergoing TAP reconstruction.
The DASH questionnaire has been developed by the American Academy of Orthopedic Surgeons (28) and has been utilized to evaluate shoulder function after LD breast reconstruction with varying results. The patients are assigned scores corresponding to their answers were 0 represents no disability and a 100 represents total disability. Two prospective studies found no statistical or clinical difference before and 1 or 3 years postoperatively (10,18) and one prospective found a significant increase in DASH score from 2.74 to 13.8 at 12-month follow-up (20). One study found significantly higher average DASH score compared to a control group, but the mean DASH score for the LD group was 16.5, which corresponds to mild impairment (15). Two retrospective studies without controls found mean DASH scores of 7.2 and 16.0, that were assessed to be low, and concluded that LD reconstruction led to minimal subjective functional disability (11,19). These retrospective studies are limited by the nature of their design and cannot consider any impairment that might have existed prior to reconstruction.
Brumback et al. and Fraulin et al. used non-commercialized questionnaires and found that 40% and 33% complained of some degree of shoulder limitations although they first study primarily attributed the complaints to tightness of axillary or back (8,9).
Another aspect of the patient’s perception of the procedure is related to whether denervation of the thoracodorsal nerve has been performed or not. Previous studies have shown relatively high incidence-rates of involuntary contractions of the reconstructed breast or jumping breasts (29), although to our knowledge, no studies have investigated any correlation between discomfort associated with breast contractions and functional impairment.
No systematic decrease in the patient’s self-reported ability to perform activities of daily living has been documented in previous reports. This may be attributable to the possible compensation from the shoulder agonists for the movements to which the LD muscle contributes.
Other aspectsOther Section
- Introduction
- Surgical technique
- Range of motion (ROM)
- Shoulder strength
- Patient-reported shoulder function
- Other aspects
- Conclusions
- Acknowledgments
- Footnote
- References
Perioperative optimization and hospitalization
A well-documented benefit of LD breast reconstruction is the possibility for introduction of Enhanced Recovery After Surgery programs which provides the possibility of short postoperative hospitalisation and is associated with low perioperative complication-rates (30). Breast reconstruction with an LD flap has previously been shown to be possible in an ambulatory setting, if an extensive out-patient system is established and cooperation with an in-hospital ward is present, should complications arise (31). A postoperative length of stay (LOS) of 3–4 days have been shown in departments without the need for specialized out-patient centers (30). This advantage offers the possibility of a shorter hospital stay, which may be associated with a positive impact on quality of life (32).
Surgical refinement
In recent years, a trend toward perforator-based flaps has emerged and while the DIEP is well established as the gold standard for autologous breast reconstruction, the use of the LD flap continues to be the first-choice alternative for many surgeons. Since its introduction in the early 90’s, the TDAP (33) flap for breast reconstruction has been gaining increasing popularity as an alternate flap originating from the back. Like the case for different myocutaneous/perforator flap pairs such as the transverse rectus abdominis myocutaneous (TRAM)/DIEP flaps, it is tempting to seek out muscle-sparing alternatives. The preference of the TAP flap by many surgeons may be contributable to the intuitively sensible in avoiding transplantation of a muscle when it is not necessary. As mentioned above, Rindom et al. demonstrated positive effects of the TDAP flap regarding shoulder-related donor-site morbidity, which favors the choice over conventional LD (17). Hamdi et al. likewise reported minimal donor-site morbidity following TDAP flap reconstruction, but had no basis for comparison with the LD flap (34). Nonetheless a total transition to perforator-based flap has not been seen despite that the TDAP-flap has been an option for almost 30 years. Even though the distinct difference between different variations of the LD-flap and the TDAP-flap lies in the absence of muscle transfer during TDAP-flap reconstruction, there are several other factors that should be considered when planning reconstructive modality and informing the patients ahead of surgery. First of all, as a perforator-based flap, success is highly dependent on surgical expertise and experience in locating the right perforator (which requires equipment in form of ultrasound or doppler verification) and assessing the viability of how big a reconstruction the given perforator can support. Secondly, in patients with comorbidities, the use of the LD flap has been advocated as the safer alternative (35). Furthermore, ERAS programs for patients undergoing reconstruction with the TDAP flap, has not been published and postoperative LOS traditionally has been reported with a median of 7 days, although an LOS of down to 2 days was demonstrated, which illustrates a potential for shorter hospitalizations in standardized settings (24). A common challenge for these patients has traditionally been the relatively large drain output that is associated with the placement of a synthetic or allogenic mesh, which necessitates hospitalisation if the department does not have a well-established plan for discharging patients with drains, although the introduction of procedures omitting the use of a mesh or using a low-irritant mesh (i.e., a vicryl mesh) may reduce the drain output drastically, thereby allowing early drain-removal.
ConclusionsOther Section
- Introduction
- Surgical technique
- Range of motion (ROM)
- Shoulder strength
- Patient-reported shoulder function
- Other aspects
- Conclusions
- Acknowledgments
- Footnote
- References
The LD flap remains a safe and reliable option for breast reconstruction. The heterogeneity of the studies regarding measurement methods, reporting outcomes, follow-up time, adjuvant therapies administered to the patients and timing of the procedure makes a relevant comparison of the studies difficult and warrants long-term prospective studies of the patients, starting at the time before any surgical procedures affecting the shoulder area. The LD flap may still be considered a viable option for breast reconstruction but further comparative studies on benefits and drawbacks of both the LD and the TDAP flap should be encouraged.
As many things in life, the raising of a latissimus dorsi flap for breast reconstruction comes with a cost as the price to pay for an LD flap may be a considerable loss of measurable shoulder strength of up to 40% for some motions. Nonetheless, the patient’s ability to perform activities of daily living does not seem to be radically impaired, leaving the price the patients perceived by the patients, lower than else expected.
AcknowledgmentsOther Section
- Introduction
- Surgical technique
- Range of motion (ROM)
- Shoulder strength
- Patient-reported shoulder function
- Other aspects
- Conclusions
- Acknowledgments
- Footnote
- References
Funding: None.
FootnoteOther Section
- Introduction
- Surgical technique
- Range of motion (ROM)
- Shoulder strength
- Patient-reported shoulder function
- Other aspects
- Conclusions
- Acknowledgments
- Footnote
- References
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard and Jørn Bo Thomsen) for the series “Breast Reconstruction - The True Multidisciplinary Approach” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-30/rc
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-30/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-30/coif). The series “Breast Reconstruction - The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- Surgical technique
- Range of motion (ROM)
- Shoulder strength
- Patient-reported shoulder function
- Other aspects
- Conclusions
- Acknowledgments
- Footnote
- References
- Tansini I. Sopra il mio nuovo processo sli amputazione della mammella. Gaz Med Ital 1906;57:141.
- McCraw JB, Penix JO, Baker JW. Repair of major defects of the chest wall and spine with the latissimus dorsi myocutaneous flap. Plast Reconstr Surg 1978;62:197-206. [Crossref] [PubMed]
- 2020 [updated 25/3/2019; cited 2021 February 9th]. Available online: https://www-dep.iarc.fr/NORDCAN/DK/StatsFact.asp?cancer=200&country=208
- Wilkins EG, Cederna PS, Lowery JC, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg 2000;106:1014-25; discussion 1026-7. [Crossref] [PubMed]
- Yun JH, Diaz R, Orman AG. Breast Reconstruction and Radiation Therapy. Cancer Control 2018;25:1073274818795489. [Crossref] [PubMed]
- Hoejvig JH, Pedersen NJ, Gramkow CS, et al. Delayed two-stage breast reconstruction: The impact of radiotherapy. J Plast Reconstr Aesthet Surg 2019;72:1763-8. [Crossref] [PubMed]
- Russell RC, Pribaz J, Zook EG, et al. Functional evaluation of latissimus dorsi donor site. Plast Reconstr Surg 1986;78:336-44. [Crossref] [PubMed]
- Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am 1992;74:377-82. [Crossref] [PubMed]
- Fraulin FO, Louie G, Zorrilla L, et al. Functional evaluation of the shoulder following latissimus dorsi muscle transfer. Ann Plast Surg 1995;35:349-55. [Crossref] [PubMed]
- Glassey N, Perks GB, McCulley SJ. A prospective assessment of shoulder morbidity and recovery time scales following latissimus dorsi breast reconstruction. Plast Reconstr Surg 2008;122:1334-40. [Crossref] [PubMed]
- Saint-Cyr M, Nagarkar P, Schaverien M, et al. The pedicled descending branch muscle-sparing latissimus dorsi flap for breast reconstruction. Plast Reconstr Surg 2009;123:13-24. [Crossref] [PubMed]
- Forthomme B, Heymans O, Jacquemin D, et al. Shoulder function after latissimus dorsi transfer in breast reconstruction. Clin Physiol Funct Imaging 2010;30:406-12. [Crossref] [PubMed]
- de Oliveira RR, do Nascimento SL, Derchain SFM, et al. Immediate breast reconstruction with a Latissimus dorsi flap has no detrimental effects on shoulder motion or postsurgical complications up to 1 year after surgery. Plast Reconstr Surg 2013;131:673e-80e. [Crossref] [PubMed]
- Garusi C, Manconi A, Lanni G, et al. Shoulder function after breast reconstruction with the latissimus dorsi flap: A prospective cohort study - Combining DASH score and objective evaluation. Breast 2016;27:78-86. [Crossref] [PubMed]
- van Huizum MA, Hoornweg MJ, de Ruiter N, et al. Effect of latissimus dorsi flap breast reconstruction on the strength profile of the upper extremity. J Plast Surg Hand Surg 2016;50:202-7. [Crossref] [PubMed]
- Sowa Y, Morihara T, Kushida R, et al. Long-term prospective assessment of shoulder function after breast reconstruction involving a latissimus dorsi muscle flap transfer and postoperative radiotherapy. Breast Cancer 2017;24:362-8. [Crossref] [PubMed]
- Rindom MB, Gunnarsson GL, Lautrup MD, et al. Shoulder-related donor site morbidity after delayed breast reconstruction with pedicled flaps from the back: An open label randomized controlled clinical trial. J Plast Reconstr Aesthet Surg 2019;72:1942-9. [Crossref] [PubMed]
- Button J, Scott J, Taghizadeh R, et al. Shoulder function following autologous latissimus dorsi breast reconstruction. A prospective three year observational study comparing quilting and non-quilting donor site techniques. J Plast Reconstr Aesthet Surg 2010;63:1505-12. [Crossref] [PubMed]
- Paolini G, Amoroso M, Pugliese P, et al. Functional sequelae following bilateral mastectomy and immediate reconstruction with latissimus dorsi flap: medium-term follow-up. J Plast Surg Hand Surg 2014;48:99-103. [Crossref] [PubMed]
- Yang JD, Huh JS, Min YS, et al. Physical and Functional Ability Recovery Patterns and Quality of Life after Immediate Autologous Latissimus Dorsi Breast Reconstruction: A 1-Year Prospective Observational Study. Plast Reconstr Surg 2015;136:1146-54. [Crossref] [PubMed]
- Højvig JB, Bonde CT. Breast reconstruction using a latissimus dorsi flap after mastectomy. Dan Med J 2015;62:A5155. [PubMed]
- Hokin JA. Mastectomy reconstruction without a prosthetic implant. Plast Reconstr Surg 1983;72:810-18. [Crossref] [PubMed]
- Schwabegger AH, Harpf C, Rainer C. Muscle-sparing latissimus dorsi myocutaneous flap with maintenance of muscle innervation, function, and aesthetic appearance of the donor site. Plast Reconstr Surg 2003;111:1407-11. [Crossref] [PubMed]
- Gunnarsson GL, Holm J, Duus N, et al. Propeller TAP flap breast reconstruction: A simplified surgical technique. J Plast Reconstr Aesthet Surg 2018;71:1424-31. [Crossref] [PubMed]
- Steffenssen MCW, Kristiansen AH, Damsgaard TE. A Systematic Review and Meta-analysis of Functional Shoulder Impairment After Latissimus Dorsi Breast Reconstruction. Ann Plast Surg 2019;82:116-27. [Crossref] [PubMed]
- Shah A, Rowlands M, Patel A, et al. Ubersense: using a free video analysis app to evaluate and improve microsurgical skills. Plast Reconstr Surg 2014;134:338e-9e. [Crossref] [PubMed]
- Stark T, Walker B, Phillips JK, et al. Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: a systematic review. PM R 2011;3:472-9. [Crossref] [PubMed]
- Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) corrected. The Upper Extremity Collaborative Group (UECG) Am J Ind Med 1996;29:602-8. [Crossref] [PubMed]
- Schroegendorfer KF, Hacker S, Nickl S, et al. Latissimus dorsi breast reconstruction: how much nerve resection is necessary to prevent postoperative muscle twitching? Plast Reconstr Surg 2014;134:1125-9. [Crossref] [PubMed]
- Højvig JH, Kehlet H, Bonde CT. Enhanced recovery after breast reconstruction with a pedicled Latissimus Dorsi flap-A prospective clinical study. J Plast Reconstr Aesthet Surg 2020; Epub ahead of print. [Crossref] [PubMed]
- Stein MJ, Frank SG, Lui A, et al. Ambulatory latissimus dorsi flap breast reconstruction: A prospective cohort study of an enhanced recovery after surgery (ERAS) protocol. J Plast Reconstr Aesthet Surg 2019;72:1950-5. [Crossref] [PubMed]
- Kishawi D, Wozniak AW, Mosier MJ. TBSA and length of stay impact quality of life following burn injury. Burns 2020;46:616-20. [Crossref] [PubMed]
- Angrigiani C, Grilli D, Siebert J. Latissimus dorsi musculocutaneous flap without muscle. Plast Reconstr Surg 1995;96:1608-14. [Crossref] [PubMed]
- Hamdi M, Salgarello M, Barone-Adesi L, et al. Use of the thoracodorsal artery perforator (TDAP) flap with implant in breast reconstruction. Ann Plast Surg 2008;61:143-6. [Crossref] [PubMed]
- Thomsen JB, Rindom MB, Rancati A, et al. Thoracodorsal artery flaps for breast reconstruction-the variants and its approach. Arch Plast Surg 2021;48:15-25. [Crossref] [PubMed]
Cite this article as: Højvig JH, Bonde CT. Narrative review of breast reconstruction with a latissimus dorsi flap—is there a price to pay? Ann Breast Surg 2022;6:24.