
The propeller thoracodorsal artery perforator flap—designs for breast reconstruction and perspectives
The latissimus dorsi flap without muscle or the thoracodorsal artery perforator (TDAP) flap was introduced by Angrigiani in 1995 (1). This was the first time the TDAP flap was used for breast reconstruction. The TDAP in one variant from a range of TDAP flaps: (I) the extended lattisimus dorsi flap (ELD), (II) the latissimus dorsi flap (LD), (III) the muscle sparing latissimus dorsi flap (MSLD), (IV) the propeller thoracodorsal artery perforator flap (pTDAP) and (V) the classic thoracodorsal artery perforator flap (cTDAP). The different indications for use of these flaps in breast reconstruction have recently been described as well as the different designs of these flaps (2). The pTDAP itself can be designed in many different ways, the location, orientation and size and outline of the skin island. The rotation/tranposition of the pedicle as well as ways to use the flap for shaping, draping and augmentation in the recipient site can also be designed in multitude of different ways. The pTDAP is often used instead of the LD flap for breast reconstruction to leave the upper body’s largest muscle intact and to avoid the possible morbidity of the shoulder and arm associated with the use of the LD muscle (3,4). The indications for using the pTDAP flap for breast reconstruction is similar to the indications for using the LD flap (2,5). The flaps can be used for both immediate and delayed breast reconstructions and often in women who previously had radiation therapy to the chest, where the damaged unpliable skin and subcutis can be replaced by the more pliable flap tissue, which enables a better cosmetic and functional result in the long run (6). The aim of this paper is to describe and illustrate different pTDAP flap designs for breast reconstruction and the perspectives.
Indication for breast reconstruction
Delayed breast reconstruction
The pTDAP is used to add extra tissue to enable the breast reconstruction and often in combination with an implant. The skin and subcutis or the remainders of the mastectomy flap in the anterior part of the thorax is raised as a musculocutaneous flap with the pectoralis major muscle. In a few cases, where the subcutis is particularly thick or if the radiation damage to the muscle is severe, the flap can be raised as a cutaneous flap without muscle (6-8). The two flaps are combined to shape and drape the reconstruction.
Immediate breast reconstruction
In women who previously have had radiation therapy in combination with a lumpectomy, the skin of the lower quadrants is often removed after the mastectomy or as part of the mastectomy specimen. The aim is to replace the tissue damaged by radiation therapy by the pliable tissue of the pTDAP (6). It sometime seems odd to excise skin and tissue which at a first glance seems unaffected by radiation, but there is a substantial risk of capsular contracture in cases, where the damaged breast skin in not replaced by pliable skin. The alternative to the pTDAP in these cases is multiple fat graftings of the radiated mastectomy flaps, however in many cases it is only a matter of time before the contractures calls for a flap solution.
The pTDAP can also be used for partial breast reconstruction or salvage of reconstructed breasts (6).
Designs
Overall, there are three steps to designing a breast using a pTDAP flap: (I) flap design, (II) axilla design and (III) breast design. The designs are described below.
Flap design
In unilateral cases the flap is raised with the patient in the lateral position. The flap can be raised simultaneously with dissection of the axilla and recipient site (7,9). In bilateral cases, the recipient site is prepared first with the patient in the supine position. The patient is then turned to the prone position to raise the flaps and subsequently turned once again to the supine position for the breast reconstruction (10).
The skin island for the TDAP flap is harvested from the back and can be designed in many different ways. The base of the skin island is marked above and around the perforator(s). The flap can be designed in various ways and in different angles, however, there are three main designs: (I) horizontal (H), (II) oblique upwards (OU) and (III) oblique downwards (OD) (9,11-14), Figure 1A.
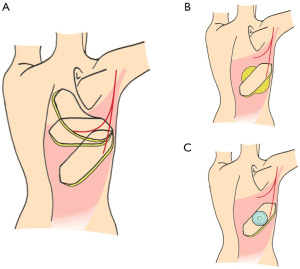
The scars of the first two options, H and OU can be hidden under clothing, whereas the third option leaves a scar in the lower part of the back, which can be difficult to hide. The flaps of the H and OU designs have to be rotated 180° or more to the recipient site, whereas the OD design only needs to be rotated in angle of 120–135° (9,11,12,15). Thus, the pedicles of the H and OU designs needs to be dissected more thoroughly and often all the way through the muscle to enable relocation of the flap from the donor site to the recipient site (9,12,15). The flap length of the oblique flap designs, the OU and the OD, can be up to 35 cm long compared to 25 cm of the H design (11). However, this also means that the distal part of the OU/OD flaps can only be used if the blood supply is reliable, when tested by fluorescence using indocyanine green or similar techniques (16). The distal 5 cm of the tip of the flap often has to be removed due to insufficient perfusion (2). The size of the flap, thickness, width and length, depends on the size and proportions of the individual patient. In skinny patients, the flap width can usually only be 6–7 cm compared to 10–11 cm in larger patients. The lengths of the flaps may variate from 18 cm up to almost 40 cm in large patients (7). The thickness and amount of subcutaneous tissue to be harvested varies a lot depending on BMI and the looseness of the skin. The largest flaps can be harvested in massive weight loss patients, where the tissue is often very loose and the perforators are relatively sizable compared to patients who have not lost weight. The flaps can be harvested as extended flaps including as much of the subcutaneous tissue under and adjacent to the skin island as possible (6,17,18). This can be designed in different ways, however in many patients the location of the subcutaneous fatty tissue allows for a “Saturn” design of the flap as illustrated in Figure 1B. The harvest of the subcutaneous tissue is often limited by the fascia of Scarpae, which should preferably be saved for closure of the donor site. Maybe this limitation can be overcome by pre-expansion, Figure 1C.
Raising the flap from the tip towards the base can be recommended using a scalpel in combination with bipolar cautery or a monopolar cautery. The skin is incised and bevelled outwards in the subcutis to add additional subcutaneous tissue to the flap volume if possible and necessary (7-9,14). The flap can be raised with or without the muscle fascia. The thickness of the muscle fascia variates and is often very thin almost as an epimysium. The argument for leaving the fascia intact is allegedly less seroma formation, however, regardless of the design there is no need for drains in the donor site as there is hardly any seroma formation (7). The advantage of raising the flap with the fascia is that it can be used to drape the implant when performing the reconstruction. The first three quarters of the flap can be raised very quickly until a close proximity to the perforators is reached (Figure 2), unless preoperative color Doppler ultrasound has revealed a sizable perforator outside the usual location (A, B, C in Figure 2). In the majority of cases the dominant perforator(s) will be located close to the anterior border of the latissimus dorsi muscle (E in Figure 2). However, the location and the size of the perforator variate and preoperative color Doppler ultrasound is recommended for identification of the perforators (6,10,19,20). In the majority of cases there are one to two sizeable perforators located at the most prominating anterior part of the LD muscle, approximately 8–12 cm from the top of the axilla and 3–5 cm from the anterior border of the LD muscle (7,9) (D, E in Figure 2). The dominant perforator(s) can be located more distal along the descending or the horizontal branches of the TDA. Sometimes the dominant perforator can be placed in unexpected locations due to scars, which has redirected the original blod flow to the skin (2) (A, B, C in Figure 2). In these cases, the CDU is a very good tool for a targeted approach (19). Once in a while there are no obvious dominant perforator and three or more smaller perforators have to be included in the flap pedicle (21).
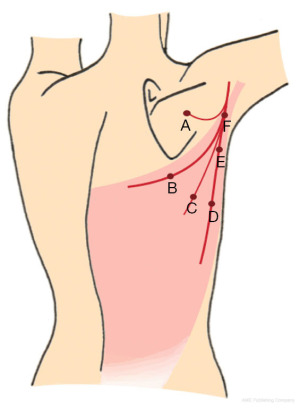
The flap area can be expanded in the back using an expander (Figure 1C), which has not been described for breast reconstruction, but for coverage of severe scarring in the cervicofacial regions (22). However, this could also be an option for breast reconstruction in patients with damaged or very thin skin in the recipient site.
The donor site is closed in three layers using absorbable sutures adapting the fascia of scarpae, the deep dermis and the subcutis. No drains are needed (7).
Axilla design
The pedicle of the pTDAP can be placed and designed in different ways depending on the location of the perforators.
- In patients where the perforator(s) is located 5–7 cm or more behind the anterior edge of the LD muscle and if the skin in the axilla is tight it is often advantageous to incise the skin between the flap and the recipient site and simply place the base of the flap in the gap to release the tight skin in the axilla and to ensure unaffected blood perfusion through the flap (10) (Figure 3A). Another reason for this approach is that the base of the flap can sometimes be quite bulky and difficult to cover by the axillary skin. The procedure not only releases the tight axillary skin, but also makes the donor site closure easier simply by providing more skin for the axilla and adjacent area, which can be somewhat tight when harvesting a big flap from the back.
- In cases where the perforator(s) perforates the muscle 3–4 cm behind the anterior edge of the LD muscle, the base of the pTDAP flap above the perforators can be deepithelialized and covered by the adjacent axillary skin (8,14). Often the skin is incised all the way from the flap to the recipient site and subsequently, when you know how much tissue is at hand the posterior base of the flap can be covered by the adjacent axillary skin in a VY manner (Figure 3B).
- When the perforator(s) is located anterior to or close the anterior edge of the LD muscle, the pTDAP can be tunneled under the axillary skin to the recipient site (Figure 3C). The tunneled base of the TDAP flap can be used to replace the missing tissue in the axilla of women who had an axillary lymph node dissection (7,14). The added tissue often leaves a satisfying cosmetic as well as functional result as the scar tissue following the axillary procedure is removed and released as part of the procedure. This often enables better movement of the shoulder and arm as perceived by the patient.
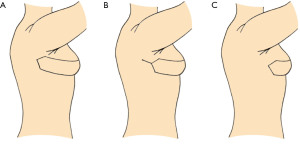
The axillary skin and base of the TDAP flap will loosen over time and in approximately 50% of cases a lateral liposuction, shape and drape procedure is needed to debulk and tighten the tissue lateral to the reconstructed breast (6,7).
Breast design
The patient is placed in the supine position for breast reconstruction. In delayed breast reconstructions, the pTDAP flap can be placed where the scar was placed following mastectomy. However, the scar is often placed quite cranially and when the relatively thick pTDAP flap is placed in the middle of the breast reconstruction with a thin tissue layer bordering both sides of the flap, this often leaves a rather abrupt transition from the mastectomy flaps to the pTDAP flap simply due to the differences in flap thickness (8,14). This can successfully be corrected by several additional fat grafting procedures (23).
However, the pTDAP flap can also be placed in the neo-inframammary crease, where it is much easier to make a smooth transition for pTDAP flap to mastectomy flap (7,10,18). The mastectomy flap is raised as a musculocutaneous flap with the major pectoralis muscle, which makes the thickness of the flap similar to the pTDAP and the extended subcutis of the pTDAP flap can be used to shape a smoother transition between the flaps (7). In cases where the mastectomy scar is placed in the lower part of the chest, the skin between the neo-inframammary crease and the mastectomy scar can be removed to avoid a third scar. However, when the scar is placed more cranially, the skin is needed for the breast reconstruction leaving three scars, the mastectomy scar, the cranial pTDAP scar and the caudal pTDAP/neo-inframammary crease scar. The latter procedure enables the best possibility for shaping and draping the reconstructed breast and eventually the best cosmetic result despite the additional third scar. There is a risk of insufficient blood supply to the mastectomy skin below the mastectomy scar. In a few cases, we have had to remove this skin in a secondary procedure.
Shaping and augmentation of the pTDAP breast reconstruction
Direct to implant
In many cases the pTDAP flap can be immediately combined with a permanent implant for breast reconstruction (8,9,24). The implant can be placed and supported in the desired location by use of a biologic or a synthetic mesh (8). In some cases, the extended fascia around the pTDAP flap can be used to support the implant and in those cases a mesh is not needed.
Expander to implant
In the last couple of years, we have increasingly used expanders for pTDAP reconstruction (10) (Figure 4A). One reason for this is that we are using the pTDAP for breast reconstruction in patients with lower BMIs, where an expander seems to be the safer option to ensure sufficient blood supply. Another reason is that the expansion enables a sufficient and sizable cavity for a correct sized permanent implant (9,14,24,25). The majority of pTDAP reconstructions are, however, still reconstructed by a direct to implant technique using a permanent implant (Figure 4B).
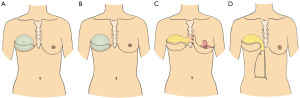
Expander-fat
When a patient wishes a breast reconstruction with a pTDAP in combination with fat grafting, the fat augmentation can preferably be combined with an expansion using an expander for preshaping the breast prior to fat grafting (23,26). In the subsequent procedures the water volume of the expander is deflated simultaneously with fat grafting of a similar amount of fat replacing the water volume in the expander with fat injected into the tissue surrounding the expander. This enables better shaping of the reconstruction and also there seems be a better take of the transplantation fat as the loose preexpanded tissue seems more susceptible to the injected fat.
Internal mammary artery perforators (IMAP)
The implants alone, in combination with fat grafting or fat grafting alone has been the mainstay for augmenting the breasts reconstructed with pTDAP flaps. However, there are other novel options, which can also be used. The pTDAP can be combined with other flaps for augmentation and added volume.
In unilateral breast reconstructions, where the patient has a large contralateral breast, which needs to be reduced, the deepithelialised skin and subcutis can be utilized as a flip over flap based on IMAPs (Figure 4C). This is only possible if a sizable perforator(s) can be identified in intercostal spaces of the caudal part of the sternum. Preoperative imaging, MRI, CDU and preferably a combination of these two imaging modalities is mandatory for preoperative identification of the perforators, which enables a targeted reconstructive procedure (19,20). When raising the IMAP flap consisting of skin and subcutaneous fat, the plastic surgeon has to be experienced in finding the correct dissection plane between the glandular tissue and the subcutaneous tissue (27). The procedure is not intended to be a breast-sharing technique, but rather to use a cutaneous flap for augmentation in order to avoid transposition of glandular tissue from one breast to the other. The flap perfusion needs to be examined by indocyanine green to ensure sufficient blood flow in the distal part of the IMAP flap prior to tunneling of the flap to the recipient site (16). The flap can be placed and used for augmentation of the cranial part of the breast reconstruction and the shaping of the flap can be supported by a reabsorbable mesh. The combined pTDAP/IMAP flaps can be augmented further by fat grafting in subsequent procedure(s) along with the lateral correction of the pTDAP pedicle (7,23). A total of 3–4 fat graftings should be anticipated, when using this approach. The bulkiness of the pedicles of both the TDAP and IMAP flaps needs to be corrected by liposuction in one of these subsequent procedures to finalize and shape the reconstruction and areas adjacent to the reconstruction.
Superior epigastric artery perforator (SEAP)
In patients with abundant loose abdominal subcutaneous tissue, who are candidates for a vertical abdominoplasty, the vertical SEAP flap can be used to augment the breast reconstruction in combination with pTDAP flap (Figure 4D). The location and size of the SEAP perforator has to be confirmed by CDU prior to surgery for a targeted procedure (19,20). The length of the flap goes from a couple of centimetres above the xiphoid process to 3–5 cm cranial to the umbilicus and the width from the midline and laterally based on a pinch test using the usual markup for a vertical abdominoplasty. The perfusion has to be checked by ICG prior to tunneling of the flap to the recipient site (16). The combined TDAP/SEAP flaps needs to be augmented by fat grafting, shaped and corrected in the same manner as the TDAP/IMAP flaps (6,7,23).
Free TDAP
The pTDAP can also be combined with a free TDAP as stacked flaps breast reconstruction (2).
Perspectives
We expect that the use of the pTDAP in combination with expanders, implants and fat for breast reconstruction will increase in the years to come. We also expect to see an increase in the use of combined flaps from the thorax, pTDAP, IMAP and SEAP, for breast reconstruction in a selected group of patients. Preexpansion of the pTDAP in the back prior to breast reconstruction is also an obvious possibility in selected cases.
Conclusions
The pTDAP can and should be designed, targeted and adapted to the individual patient when used for breast reconstruction. This entails the flap size and shape in the back, the choice and use of perforators, the design and rotation in the axilla and the breast reconstruction when using the flap for augmentation, shaping and draping using expanders, implants, fat grafting or in combined with other flaps.
Acknowledgments
The authors would like to acknowledge Caroline Lilja for creating the illustrations for the paper.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Breast Surgery for the series “Breast Reconstruction - The True Multidisciplinary Approach”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-14/coif). The series “Breast Reconstruction - The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. JBT served as the unpaid Guest Editor of the series and and serves as an unpaid editorial board member of Annals of Breast Surgery from December 2019 to November 2023. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Angrigiani C, Grilli D, Siebert J. Latissimus dorsi musculocutaneous flap without muscle. Plast Reconstr Surg 1995;96:1608-14. [Crossref] [PubMed]
- Thomsen JB, Rindom MB, Rancati A, et al. Thoracodorsal artery flaps for breast reconstruction-the variants and its approach. Arch Plast Surg 2021;48:15-25. [Crossref] [PubMed]
- Rindom MB, Gunnarsson GL, Lautrup MD, et al. Shoulder-related donor site morbidity and patient-reported satisfaction after delayed breast reconstruction with pedicled flaps from the back: A comparative analysis. J Plast Reconstr Aesthet Surg 2018;71:1108-15. [Crossref] [PubMed]
- Rindom MB, Gunnarsson GL, Lautrup MD, et al. Shoulder-related donor site morbidity after delayed breast reconstruction with pedicled flaps from the back: An open label randomized controlled clinical trial. J Plast Reconstr Aesthet Surg 2019;72:1942-9. [Crossref] [PubMed]
- Mushin OP, Myers PL, Langstein HN. Indications and Controversies for Complete and Implant-Enhanced Latissimus Dorsi Breast Reconstructions. Clin Plast Surg 2018;45:75-81. [Crossref] [PubMed]
- Jacobs J, Børsen-Koch M, Gunnarsson GL, et al. The Versatile Extended Thoracodorsal Artery Perforator Flap for Breast Reconstruction. Ann Plast Surg 2016;77:396-400. [Crossref] [PubMed]
- Gunnarsson GL, Holm J, Duus N, et al. Propeller TAP flap breast reconstruction: A simplified surgical technique. J Plast Reconstr Aesthet Surg 2018;71:1424-31. [Crossref] [PubMed]
- Børsen-Koch M, Gunnarsson GL, Udesen A, et al. Direct delayed breast reconstruction with TAP flap, implant and acellular dermal matrix (TAPIA). J Plast Reconstr Aesthet Surg 2015;68:815-21. [Crossref] [PubMed]
- Angrigiani C, Rancati A, Escudero E, et al. Propeller thoracodorsal artery perforator flap for breast reconstruction. Gland Surg 2014;3:174-80. [PubMed]
- Lorenzen MM, Gunnarsson GL, Bille C, et al. Visualized bilateral breast reconstruction by propeller thoracodorsal artery perforator flaps. Gland Surg 2019;8:S262-70. [Crossref] [PubMed]
- Thomsen JB, Gunnarsson GL. The evolving breast reconstruction: from latissimus dorsi musculocutaneous flap to a propeller thoracodorsal fasciocutaneous flap. Gland Surg 2014;3:151-4. [PubMed]
- Hamdi M, Salgarello M, Barone-Adesi L, et al. Use of the thoracodorsal artery perforator (TDAP) flap with implant in breast reconstruction. Ann Plast Surg 2008;61:143-6. [Crossref] [PubMed]
- Hamdi M, Van Landuyt K, Hijjawi JB, et al. Surgical technique in pedicled thoracodorsal artery perforator flaps: a clinical experience with 99 patients. Plast Reconstr Surg 2008;121:1632-41. [Crossref] [PubMed]
- Thomsen JB, Bille C, Wamberg P, et al. Propeller TAP flap: is it usable for breast reconstruction? J Plast Surg Hand Surg 2013;47:379-82. [Crossref] [PubMed]
- Hamdi M, Van Landuyt K, Monstrey S, et al. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg 2004;57:531-9. [Crossref] [PubMed]
- Alstrup T, Christensen BO, Damsgaard TE. ICG angiography in immediate and delayed autologous breast reconstructions: peroperative evaluation and postoperative outcomes. J Plast Surg Hand Surg 2018;52:307-11. [Crossref] [PubMed]
- Gunnarsson GL, Børsen-Koch M, Nielsen HT, et al. Bilateral Breast Reconstruction with Extended Thoracodorsal Artery Perforator Propeller Flaps and Implants. Plast Reconstr Surg Glob Open 2015;3:e435. [Crossref] [PubMed]
- Angrigiani C, Rancati A, Escudero E, et al. Extended thoracodorsal artery perforator flap for breast reconstruction. Gland Surg 2015;4:519-27. [PubMed]
- Gunnarsson GL, Tei T, Thomsen JB. Color Doppler Ultrasonography-Targeted Perforator Mapping and Angiosome-Based Flap Reconstruction. Ann Plast Surg 2016;77:464-8. [Crossref] [PubMed]
- Ibrahim RM, Gunnarsson GL, Akram J, et al. Color Doppler ultrasonography targeted reconstruction using pedicled perforator flaps-a systematic review and meta-analysis. Eur J Plast Surg 2018;41:495-504. [Crossref] [PubMed]
- Angrigiani C, Rancati A, Artero G, et al. TDAP: Island versus propeller. J Plast Reconstr Aesthet Surg 2016;69:506-11. [Crossref] [PubMed]
- Wang AW, Zhang WF, Liang F, et al. Pre-expanded thoracodorsal artery perforator-based flaps for repair of severe scarring in cervicofacial regions. J Reconstr Microsurg 2014;30:539-46. [Crossref] [PubMed]
- Kristensen RN, Gunnarsson GL, Børsen-Koch M, et al. Fast and simple fat grafting of the breast. Gland Surg 2015;4:572-6. [PubMed]
- Adler N, Seitz IA, Song DH. Pedicled thoracodorsal artery perforator flap in breast reconstruction: clinical experience. Eplasty 2009;9:e24. [PubMed]
- Bank J, Ledbetter K, Song DH. Use of thoracodorsal artery perforator flaps to enhance outcomes in alloplastic breast reconstruction. Plast Reconstr Surg Glob Open 2014;2:e140. [Crossref] [PubMed]
- Manconi A, De Lorenzi F, Chahuan B, et al. Total Breast Reconstruction With Fat Grafting After Internal Expansion and Expander Removal. Ann Plast Surg 2017;78:392-6. [Crossref] [PubMed]
- Bille C, Dalaei F, Thomsen JB. Identifying the dissection plane for mastectomy-description and visualization of our technique. Gland Surg 2019;8:S276-80. [Crossref] [PubMed]
Cite this article as: Thomsen JB, Børsen-Rindom M, Rancati A, Angrigiani C. The propeller thoracodorsal artery perforator flap—designs for breast reconstruction and perspectives. Ann Breast Surg 2022;6:22.

