
A comprehensive treatment algorithm for patients requiring simultaneous breast and lymphedema reconstruction based on lymph node transfer
IntroductionOther Section
Lymphedema is a chronic and progressive disease, which is characterized by insufficient lymph transport, resulting in accumulation of high protein fluid in the interstitial space. It is accompanied by irreversible structural changes that lead to edema of the limbs or other parts of the body (1). Most secondary lymphedema cases in Europe and USA are associated with breast-cancer treatment; the incidence varies from 6% to 60% among studies, depending on the diagnostic method and follow-up time (2-4), while in patients who underwent breast reconstruction, the incidence of breast cancer related lymphedema was found decreased (5,6). The gold standard in breast restoration remains the autologous based reconstruction especially in radiotherapy patients (6-8). Although free abdominal flaps provide an excellent and aesthetic pleasant breast, latissimus dorsi reconstruction offers a reliable alternative (9-11).
Among treatment modalities used for secondary upper limb lymphedema, the efficacy of autologous free vascularized lymphatic tissue transfer, has been well established and the procedure has been adapted at the treatment guidelines of the International Society of Lymphology (12-16). Technically, lymph nodes are harvested from a donor site and transplanted to the lymphedematous limb; inguinal, submental, supraclavicular and lateral thoracic lymph nodes represent the most popular donor sites (17). Becker described this technique as a logical reconstructive approach to treat lymphedema disease (17); the flap bridges the injured and interrupted lymphatic pathways and reestablishes the lymphatic flow by promoting lymphangiogenesis (17-19). These transplanted lymphatic tissues have shown long term successful functionality, based on radiotracer uptake in postoperative lymphoscintigraphy (20).
Saaristo et al., were the first who published their outcomes on lymph node transfer associated to microvascular breast reconstruction and documented the effectiveness of this combined procedure in terms of simultaneous breast and lymphedema reconstruction in a single operation; they also demonstrated that vascularized lymph nodes, promote the lymphangiogenesis by increasing growth factors, such as vascular endothelial growth factor-C (19).
Thereafter, several studies have described the benefits of simultaneous breast and secondary upper-limb lymphedema reconstruction, with most of them using a chimeric deep inferior epigastric perforator (DIEP) flap coupled to an inguinal lymph nodal flap (19,21-24). The use of the internal mammary over the thoracodorsal vessels has gained in popularity as recipient vessels in breast reconstruction cases, while lateral thoracic or thoracodorsal vascular pedicles are frequently used for revascularization of free autologous lymph node transplantation (18,25-27). Another well-established surgical technique, the latissimus dorsi flap combined with lymph nodes (LD-LNT) has also been described as a reliable alternative procedure for reconstructing mastectomy and lymphedema patients (25).
In the present study, we describe our algorithmic approach to guide the decision-making in these combined breast and lymphedema reconstructive procedures and, specifically, the selection of the appropriate breast reconstruction technique associated with vascularized lymph tissue transfer, taking into consideration the stage of the upper-limb lymphedema, the patient’s characteristics, and our long-term results. We present the following article in accordance with the STROBE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-20-142/rc).
MethodsOther Section
Preoperative evaluation
All patients requiring a simultaneous breast and lymphedema reconstruction should undergo a thorough history, physical and imaging examination.
Data collection include the type of breast cancer, type of surgery (partial or total mastectomy, previous breast reconstruction), number of excised lymph nodes, details of additional therapy (chemo-, radiotherapy). Additional documentation for lymphedema includes the onset of symptoms (feeling of heaviness, pain, number of infection episodes per year), the need of oral or intravenous antibiotics, working abilities, quality of life assessment and previous lymphedema management with conservative or surgical methods. Medical history also includes any other condition which may influence the decision making for breast and lymphedema reconstruction, such as coagulopathy, obesity, smoking habits, psychiatric disorder, disability to follow rehabilitation protocols etc.
Regarding physical examination, the mastectomy and the lymphedema sites are examined individually. The quality of mastectomy skin is evaluated for elasticity and softness; radiation skin damage is marked, if any. In thin dry skin, or in cases with skin radiotherapy damages, we always consider an autologous tissue reconstruction. The lymphedema arm and axilla are assessed to perceive the severity of the disease and the volume difference between the affected and non-affected limb, in order to classify the stage of the disease according to International Society of Lymphology (16).
In authors’ institution algorithm, the preoperative imaging examinations include a three-dimensional computed tomography (3DCT) abdominal angiogram, to identify the vascular anatomy of the DIEP vessels and predesign the abdominal flap, a single-photon emission computed tomography/computed tomography (SPECT/CT) lymphoscintigraphy of the upper limbs for diagnosis, as well as of the lower limbs to delineate and select the most functional donor site lymph nodes, a fluoroscopic indocyanine green (ICG) superficial lymphangiography, and a lympho-magnetic resonance imaging (MRI) (20,28,29). SPECT/CT also investigates the presence of functional lateral thoracic lymph nodes, which are situated ipsilateral to the affected lymphedema limb. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Scientific Committee of 304/31.10.2018 and informed consent was taken from all the patients. Regarding the post-operative follow-up, patients are evaluated for wound healing in a weekly basis up to 3–6 weeks, following by a regular 3, 6, 12 months and then yearly follow-up appointments for volume measurements and quality of life assessment.
Decision making algorithm in simultaneous breast and lymphedema reconstruction
Our algorithmic approach considers the type of breast reconstruction, as the first step, followed by the selection of the appropriate lymphatic treatment (Table 1).
Table 1
| Distinctive features “Breast” | Distinctive features “Lymphedema” & other characteristics | Surgery | |
|---|---|---|---|
| Partial mastectomy | |||
| Inner breast defect | Stage 1, 2, 3 | If LT LN present | Lipofilling + pedicle LT LNT + CS @ upper limb |
| If LT LN absent | Lipofilling + free groin LNT @ axilla + CS @ upper limb | ||
| Outer breast defect | If LT LN present | T-DAP + LT LNT @ axilla + CS @ upper limb | |
| If LT LN absent | T-DAP + groin LNT @ axilla + CS @ upper limb | ||
| Total mastectomy | Stage 1, 2, 3 | Adequate abdominal tissue | DIEP + groin LNT @ axilla + CS @ upper limb |
| “Pear shaped body” | FALD + groin LNT @ axilla + CS @ upper limb | ||
| Chest skin soft and adequate skin/adipose flaps | 2 stage alloplastic breast reconstruction (expander & later implant) + groin LNT @ axilla + CS @ upper limb | ||
LT LN, lateral thoracic lymph nodes; LNT, lymph node transfer; CS, collagen scaffolds; T-DAP, thoracodorsal artery perforator; DIEP, deep inferior epigastric perforator; FALD, fat augmented latissimus dorsi. Stage 1–3 according to ISL (16).
Partial breast reconstruction
If the defect concerns the outer quadrants of the breast, we suggest a thoracodorsal artery perforator flap (TDAP), while for inner breast defects, lipofilling, in one or more sessions, is our recommendation.
Total breast reconstruction
In cases with available abdominal tissues with no previous abdominal surgeries, a free abdominal based breast reconstruction is the first choice.
In thin nulliparous patients, with adequate adipose tissue on the thighs or buttocks (pear shaped body), we suggest an autologous breast reconstruction using the fat-augmented latissimus dorsi (FALD) myocutaneous flap.
Only in women with soft chest skin, without history of radiotherapy, and early stage lymphedema, we may consider an alloplastic two-stage breast reconstruction.
Lymphedema management
Stage 1
If the ipsilateral lateral thoracic (LT) lymph nodes are present and functional at the preoperative SPECT/CT images, a pedicled LT lymph node transfer (LNT) is recommended. If collagen scaffolds (CS) are available, they may be inserted subcutaneously and attached to the flap towards the upper limb, as an additional agent to enhance lymphangiogenesis. The use of a pedicled LT lymph node flap may be associated to a pedicled latissimus dorsi or TDAP flap, when indicated.
If ipsilateral lateral thoracic (LT) lymph nodes are not available, we suggest the use of a free inguinal lymph node transfer (LNT); the inguinal lymph node flap is placed in the affected axilla after complete scar release, and revascularized to the lateral thoracic or serratus anterior vessels. Collagen scaffolds may also be used.
In case that a Stage 1 lymphedema patients are good candidates for a total breast reconstruction with a free abdominal flap, we always use inguinal LNT, and do not examine the LT lymph nodes.
Stage 2 and 3
In more advanced stages a free vascularized LNT is recommended at the axilla, with or without CS at the upper limb, considering the ICG-based presence of functional lymphatics. In cases with advanced lymphedema, liposuction or lipectomy of the upper limb may be considered as a secondary adjunct reconstructive procedure.
Technical considerations
A TDAP flap for partial breast reconstruction is combined either with a pedicled propeller LT LNT which is based in a separate branch of the subscapular artery or the lateral thoracic artery, or with a free inguinal LNT.
In total breast reconstruction with free abdominal tissue transfer, a DIEP flap conjoined with the inguinal LNT is transferred as “en block” tissue, but always performing double anastomoses to internal mammary and thoracodorsal vessels
When a FALD flap is performed, either it is elevated with the LT lymph nodes if they are present, or it is coupled with a free inguinal lymph node flap, which is harvested as a first step of the surgery, transferred and anastomosed in the axilla; then we continue with elevating the myocutaneous latissimus dorsi flap, having the patient in a lateral position, prepare the chest skin flaps and rotate the LD flap at the chest pocket. Last, we perform the lipofilling according to our previously published technique (9).
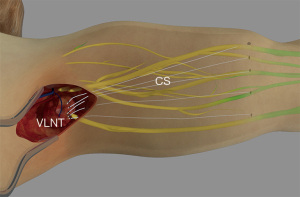
In order to select those cases that may benefit from the use of collagen scaffolds, first a real time indocyanine green fluoroscopy is performed in order to indicate if any functional lymphatics are present at the lymphedematous limb. The aim of the procedure, by implanting the collagen scaffolds subcutaneously with a suture passer, it is to connect the vascularized transferred lymphatic flap with the functional lymphatic vessels. The scaffolds are placed in the vicinity with the lymphatics, in order to augment the lymphangiogenesis (Figure 1). Duration time of five collagen thread implantation is estimated less than 10 minutes.
Outcomes
Since 2011, a total of 69 mastectomy (35 partial, 34 total) and upper limb lymphedema patients, with a mean age of 49 (range, 24–77) years and an average body mass index (BMI) of 27.8 (range, 20–35) kg/m2, were included and completed the study. Follow-up (FU) ranged from 1–9 years (mean FU period 4 years and 8 months). Table 2 summarizes the patients’ features and procedures that were performed following our algorithmic approach.
Table 2
| Lymphedema stage | Breast characteristics | Surgery | |
|---|---|---|---|
| Stage 1 (n=9), Stage 2 (n=21), Stage 3 (n=5) | Partial mastectomy (n=35) | Inner breast defect (n=25) | Lipofilling + pedicle LT LNT (n=3)/CS @ upper limb (2 of 3) |
| Lipofilling + free groin LNT @ axilla (n=22)/CS @ upper limb (1 of 22) | |||
| Outer breast defect (n=10) | T-DAP + LT LNT @ axilla + CS @ upper limb (n=1) | ||
| T-DAP + groin LNT @ axilla (n=9)/CS @ upper limb (2 of 9) | |||
| Stage 1 (n=14), Stage 2 (n=15), Stage 3 (n=5) | Total mastectomy (n=34) FALD + groin LNT @ axilla (n=8)/CS @ upper limb (1 of 8) 2 stage alloplastic breast reconstruction (expander & later implant) + groin LNT @ axilla (n=2) |
DIEP + groin LNT @ axilla (n=24)/CS @ upper limb (1 of 24) | |
LT LN, lateral thoracic lymph nodes; LNT, lymph node transfer; CS, collagen scaffolds; T-DAP, thoracodorsal artery perforator; DIEP, deep inferior epigastric perforator; FALD, fat augmented latissimus dorsi.
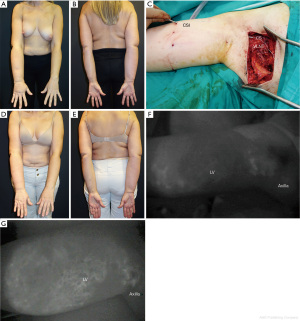
In partial mastectomy subpopulation, nine patients had Stage 1 lymphedema, 21 Stage 2 and five Stage 3. Inner breast defect patients (n=25) underwent a lipofilling procedure combined with a pedicle LT LNT (n=3), or a free vascularized groin LNT (n=22). Eighteen of those patients had more than one lipofilling sessions. Collagen scaffolds (CS) were used recently in combination with two pedicled and one free LNT. Ten outer breast defect patients underwent a T-DAP flap combined with a pedicled LT LNT and collagen scaffold implantation (n=1), and another nine had a T-DAP flap combined with a free inguinal LNT flap; from the latter, two had also collagen scaffolds implantation (Figure 2).
In total mastectomy cases, fourteen patients were classified as Stage 1 lymphedema, fifteen Stage 2, and five Stage 3. Twenty-four patients underwent a conjoined DIEP + inguinal LNT flap, eight were treated with a FALD combined with free inguinal LNT and two patients received an alloplastic reconstruction with a free inguinal LNT at the axilla. Collagen scaffolds were added in one DIEP + LNT, and in one FALD + LNT patient.
A mean of 4.1 lymph nodes were contained in the lymph node flaps, a mean volume reduction was documented as 54.8% (52.9% for Stage 1, 54.3% for Stage 2, and 61% for Stage 3), infection episodes were reduced from mean 1.2 to 0.2 per patient per year. As for the subjective symptoms in a Visual Analog Scale, all patients reported significant reduction of pain (P=0.000) and feeling of heaviness (P=0.000) of the affected extremity with significant overall functional improvement (P=0.000). The need for secondary breast symmetrization surgeries was 1.4 per patient, and upper limb suction lipectomy was performed in 8 patients.
At 12 month’s postoperative lymphoscintigraphy, evidence of radioactive transplanted lymph nodes and/or reduction of the dermal backflow were documented in 78.9% of the operated patients.
One conjoined DIEP + LNT flap partially failed, and one LNT was extirpated after a patient fall onto her arm the first post-operative day. No other major flap or donor site complication i.e., wound dehiscence, wound infection, donor site lymphedema, seromas, or lymphocoeles were recorded.
DiscussionOther Section
Conservative treatment has traditionally been considered as the method of choice for secondary lymphedema management, showing some temporary reduction in edema levels, without however offering permanent treatment; results are reversible if patients stop applying conservative methods, such as lymphatic drainage, bandage, and pressure garments (18,30). Many different surgical procedures have been proposed to alleviate lymphedema, mainly concluding that lymphatic tissue physiologic reconstructive methods, such as lymphovenous anastomosis (LVA) and LNT, represent the gold standard in the surgical management of lymphedema (17-21). Vascularized LNT has proven its efficacy in long term results, while several studies support the mechanism of lymphangiogenesis with regeneration of functional lymphatic vessels under the increased endogenous expression of vascular endothelial growth factor C (VEGF-C) (12,19,20,31). Boardman and Swartz more clearly have demonstrated that: (I) interstitial fluid channels are formed in the direction of lymph flow, and (II) VEGF-C attracts endothelial cells and, through interstitial fluid channeling, precedes and may even direct lymphangiogenesis (31). Data has shown that following axillary scar release, interstitial fluid channels are formed allowing lymph flow, while the high expression of VEGF-C, which is secreted from the vascularized lymph nodes, attracts endothelial cells and promotes lymphatic cell migration and functional lymphatic capillary organization in the direction of lymph flow (20). Thus, implantation of nanofibrillar collagen scaffolds, which initially serve as lymphatic channels, may guide lymphangiogenesis, enhance formation of functional lymphatic vessels into and in the vicinity of the scaffolds, and restore the functional integrity of the lymphatic system (32).
Regarding lymphatic flow restoration in breast-cancer related lymphedema patients, several studies have reported the feasibility, safety and benefits of the simultaneous breast and lymphedema reconstruction, demonstrating also its positive effect in patients’ quality of life (6,12,18,19,21,23,24). Saaristo et al. first reported the use of a modified free lower abdominal flap containing lymph nodes and lymphatic vessels in a series of nine mastectomy and lymphedema patients (19). In this study, upper limb perimeter decreased in 7 of 9 patients and postoperative lymphoscintigraphy showed some improvement in lymphatic vessel function. An important outcome of the study was the expression of VEGF-C in the normal human lymph nodes, and a potential application of lymphatic growth factors within the transferred lymph nodes.
Masia et al., presented the Barcelona algorithm, proposing an abdominal perforator flap DIEP or superficial inferior epigastric artery (SIEA)] coupled to an autologous lymph node flap, to manage lymphedema patients with amastia (33). Additional LVAs may be performed distally on the affected limb. In this study, the authors claim that the benefit of LVAs is observed at the area where the anastomoses have been performed. Interestingly, their results reach a plateau at 18 months, while according to our experience the LNT patients, continue to improve beyond this time frame, not only in terms of volume reduction, but also in terms of soft tissue improvement on the affected limb.
Chang et al., in a review article, advocated the potential benefit of the combined autologous abdominal free flap, that offers an aesthetic breast reconstruction, and the vascularized inguinal lymph node transfer which provides the potential to improve lymphedema in a single operation (34). The MD Anderson group introduced their standard of care procedure augmenting the autologous breast reconstruction and inguinal LNT with lymphovenous bypass (35). Specifically, patients who underwent the “Breast Reconstruction Including Lymphovenous bypass and Inguinal to Axillary Node Transfer” (BRILIANT) operation, have all demonstrated improvement in their lymphedema, within a mean follow-up of 19.1 months. For this period, none of the patients suffered from post-operative cellulitis, and most of them reported a satisfactory volume reduction.
Our rational for treating secondary lymphedema patients which is related to lymphadenectomy and/or radiation injury is to provide an “etiological” reconstruction. We first radically debride the fibrotic tissue from the axilla, and second replace the obliterated lymphatic tissue with similar healthy vascularized adipose tissue that contains lymph nodes and lymphatic vessels, to regenerate lymphatic flow through the lymphangiogenesis. We avoid to perform LVA/lymphovenous bypass (LVB) at the upper arm, in order to preserve any functional lymphatic vessel from the already reduced number of healthy lymphatic vessels. Furthermore, having available collagen scaffolds, they may augment the lymphangiogenesis guiding the lymph flow from the edematous areas towards the implanted vascularized lymphatic tissue.
In a comparative study, Akita et al., concluded that the association of a DIEP flap to a LNT in lymphedema breast-cancer patients resulted in superior outcomes compared to those who had a LNT alone (36). Moreover, our preliminary results in a yet unpublished series, demonstrated that the size of the flap plays an important role in better outcome, while the use of a combined inguinal lymph node and DIEP flap, has a positive correlation in volume reduction of the lymphedema in upper limbs.
However, a severe drawback of the inguinal LNT is the risk of iatrogenic or donor site lymphedema (DSL) (21,29); published data on review studies confirmed a potential risk for donor lower limb lymphedema up to 1.6% of the cases, following LNT in breast-cancer lymphedema patients (29). Although the incidence of postoperative DSL is low, meticulous harvesting of lymph node should be seriously considered, and new advanced techniques such as the “reverse lymphatic mapping” and the “selective lymph node technique” have to be applied with an effort to eliminate the risk of iatrogenic complication (20,37).
Although Ciudad et al., pointed out that the main advantage of the chimeric DIEP and inguinal lymph node flap is that it can be raised as a single flap and transferred en bloc to the recipient site, in order to reduce the risk of iatrogenic DSL, the authors have published an alternative reconstructive option using an autologous abdominally based free flap associated to a gastroepiploic vascularized lymph node transfer (GE-LNT) for postmastectomy lymphedema patients (21); other advantages of the GE-LNT are the reliable amount of lymphatic tissue and lymph nodes, and the ability to divide the whole flap in two isolated flaps based on the two gastroepiploic vessels in order to bridge larger defects. We believe that, a detailed anatomical knowledge of the area, the presence of an experienced laparoscopic general surgeon, a steep learning curve and a mini laparotomy should first be considered for harvesting a GE-LNT. In our algorithm, inguinal lymph nodes are the first donor-site choice for LNT, and may be coupled to all breast reconstructive methods, i.e., microvascular abdominal flap, pedicled latissimus dorsi, as well as implant-based reconstruction; using the SPECT-CT assisted “selected lymph node” technique, we minimize the risk for postoperative DSL, while avoiding the need for abdominal surgery (20,29).
As already mentioned, the free abdominal flap combined with a lymph node transfer is the most recommended procedure for breast and lymphedema reconstruction, but it is not the only approach that has been published. There have been reports of using a pedicled or free latissimus dorsi myocutaneous flap including the lateral thoracic lymph nodes (24,36). Indications for latissimus dorsi flap combined with vascularized lymph node transfer include immediate or delayed partial breast reconstructions with upper limb lymphedema, total breast reconstruction in post-irradiated patients, or breast reconstruction in cases with unavailable, previously failed, or contraindicated abdominally-based reconstruction (24,38). Inbal et al., suggested raising a conjoined pedicle latissimus dorsi myocutaneous and lateral thoracic lymph nodal flap in a single or double pedicle (24). However, this procedure is only feasible in cases of intact lateral thoracic nodes following an axillary dissection and radiotherapy. In such cases a free LD LNT is recommended, which has proven its efficacy in improving patients’ lymphedema symptoms (24). According to our algorithm, when functional local lateral thoracic lymph nodes are absent, we recommend the use of an autologous pedicled latissimus dorsi myocutaneous flap combined with a free vascularized lymph node flap, preferably harvested form the inguinal area. Supraclavicular or gastroepiploic lymph nodes may also be used, depending on the surgeons’ preferences.
Our suggested algorithm has evolved since 2011. Despite the fact that the combined DIEP with inguinal lymph nodes flap represents the gold standard procedure as it is raised in an en block tissue for this demanding reconstructive procedure, our results have documented that both DIEP or FALD flaps combined with LNT can be similarly efficacious in reducing limb volume and lymphedema symptoms, and also improving quality of patients’ life.
ConclusionsOther Section
As LNT represents an effective therapeutic approach for lymphedema patients, the combination of LNT with an autologous breast reconstruction can provide the best outcomes in a single surgical procedure in post-mastectomy lymphedema patients. The selection of DIEP or FALD flaps follows the same criteria as for breast reconstruction alone. Our algorithm might be a helpful tool in decision making, when a simultaneous breast and lymphedema reconstruction is required.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dung Nguyen) for the series “Cutting-edge of Complex Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-20-142/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-20-142/dss
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-20-142/coif). The series “Cutting-edge of Complex Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Scientific Committee of 304/31.10.2018 and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Fialka-Moser V, Korpan M, Varela E, et al. The role of physical and rehabilitation medicine specialist in lymphoedema. Ann Phys Rehabil Med 2013;56:396-410. [Crossref] [PubMed]
- Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol 2005;3:208-17. [Crossref] [PubMed]
- Brennan MJ, DePompolo RW, Garden FH. Focused review: postmastectomy lymphedema. Arch Phys Med Rehabil 1996;77:S74-80. [Crossref] [PubMed]
- Armer JM, Ballman KV, McCall L, et al. Lymphedema symptoms and limb measurement changes in breast cancer survivors treated with neoadjuvant chemotherapy and axillary dissection: results of American College of Surgeons Oncology Group (ACOSOG) Z1071 (Alliance) substudy. Support Care Cancer 2019;27:495-503. [Crossref] [PubMed]
- Card A, Crosby MA, Liu J, et al. Reduced incidence of breast cancer-related lymphedema following mastectomy and breast reconstruction versus mastectomy alone. Plast Reconstr Surg 2012;130:1169-78. [Crossref] [PubMed]
- Nguyen AT, Chang EI, Suami H, et al. An algorithmic approach to simultaneous vascularized lymph node transfer with microvascular breast reconstruction. Ann Surg Oncol 2015;22:2919-24. [Crossref] [PubMed]
- Karami RA, Ghanem OA, Ibrahim AE. Radiotherapy and breast reconstruction: a narrative review. Ann Breast Surg 2020;4:17. [Crossref]
- Bordianu A, Leoveanu I, Chang EI. Autologous breast reconstruction beyond the DIEP: a narrative review of autologous breast reconstruction options beyond the DIEP flap. Ann Breast Surg 2020;4:15. [Crossref]
- Demiri EC, Dionyssiou DD, Tsimponis A, et al. Outcomes of Fat-Augmented Latissimus Dorsi (FALD) Flap Versus Implant-Based Latissimus Dorsi Flap for Delayed Post-radiation Breast Reconstruction. Aesthetic Plast Surg 2018;42:692-701. [Crossref] [PubMed]
- Demiri EC, Tsimponis A, Pagkalos A, et al. Fat-Augmented Latissimus Dorsi versus Deep Inferior Epigastric Perforator Flap: Comparative Study in Delayed Autologous Breast Reconstruction. J Reconstr Microsurg 2021;37:208-15. [Crossref] [PubMed]
- Flaherty F, Chouhy E, Vizcay M, et al. Revisiting the back as an option in breast reconstruction, from basic to cutting edge: a narrative review. Ann Breast Surg 2020;4:16. [Crossref]
- Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg 2006;243:313-5. [Crossref] [PubMed]
- Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg 2009;123:1265-75. [Crossref] [PubMed]
- Cheng MH, Huang JJ, Wu CW, et al. The mechanism of vascularized lymph node transfer for lymphedema: natural lymphaticovenous drainage. Plast Reconstr Surg 2014;133:192e-8e. [Crossref] [PubMed]
- Chen W, McNurlen M, Ding J, et al. Vascularized lymph vessel transfer for extremity lymphedema - is transfer of lymph node still necessary? Int Microsurg J 2019;3:1. [Crossref]
- Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020;53:3-19. [PubMed]
- Becker C, Vasile JV, Levine JL, et al. Microlymphatic surgery for the treatment of iatrogenic lymphedema. Clin Plast Surg 2012;39:385-98. [Crossref] [PubMed]
- Dionyssiou D, Demiri E, Tsimponis A, et al. A randomized control study of treating secondary stage II breast cancer-related lymphoedema with free lymph node transfer. Breast Cancer Res Treat 2016;156:73-9. [Crossref] [PubMed]
- Saaristo AM, Niemi TS, Viitanen TP, et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg 2012;255:468-73. [Crossref] [PubMed]
- Dionyssiou D, Demiri E, Sarafis A, et al. Functional lymphatic reconstruction with the "Selected Lymph Node" technique guided by a SPECT-CT lymphoscintigraphy. J Surg Oncol 2019;120:911-8. [Crossref] [PubMed]
- Ciudad P, Manrique OJ, Bustos SS, et al. Combined microvascular breast and lymphatic reconstruction with deep inferior epigastric perforator flap and gastroepiploic vascularized lymph node transfer for postmastectomy lymphedema patients. Gland Surg 2020;9:512-20. [Crossref] [PubMed]
- Schaverien MV, Badash I, Patel KM, et al. Vascularized Lymph Node Transfer for Lymphedema. Semin Plast Surg 2018;32:28-35. [Crossref] [PubMed]
- Forte AJ, Cinotto G, Boczar D, et al. Lymph node transfer combined with deep inferior epigastric perforators and transverse rectus abdominis myocutaneous procedures: a systematic review. Gland Surg 2020;9:521-7. [Crossref] [PubMed]
- Inbal A, Teven CM, Chang DW. Latissimus dorsi flap with vascularized lymph node transfer for lymphedema treatment: Technique, outcomes, indications and review of literature. J Surg Oncol 2017;115:72-7. [Crossref] [PubMed]
- Moran SL, Nava G, Behnam AB, et al. An outcome analysis comparing the thoracodorsal and internal mammary vessels as recipient sites for microvascular breast reconstruction: a prospective study of 100 patients. Plast Reconstr Surg 2003;111:1876-82. [Crossref] [PubMed]
- Temple CL, Strom EA, Youssef A, et al. Choice of recipient vessels in delayed TRAM flap breast reconstruction after radiotherapy. Plast Reconstr Surg 2005;115:105-13. [PubMed]
- Hamdi M, Blondeel P, Van Landuyt K, et al. Algorithm in choosing recipient vessels for perforator free flap in breast reconstruction: the role of the internal mammary perforators. Br J Plast Surg 2004;57:258-65. [Crossref] [PubMed]
- Dionyssiou D, Demiri E, Tsimponis A, et al. Predesigned breast shaping assisted by multidetector-row computed tomographic angiography in autologous breast reconstruction. Plast Reconstr Surg 2014;133:100e-8e. [Crossref] [PubMed]
- Demiri E, Dionyssiou D, Tsimponis A, et al. Donor-Site Lymphedema Following Lymph Node Transfer for Breast Cancer-Related Lymphedema: A Systematic Review of the Literature. Lymphat Res Biol 2018;16:2-8. [Crossref] [PubMed]
- Quéré I, Presles E, Coupé M, et al. Prospective multicentre observational study of lymphedema therapy: POLIT study. J Mal Vasc 2014;39:256-63. [Crossref] [PubMed]
- Boardman KC, Swartz MA. Interstitial flow as a guide for lymphangiogenesis. Circ Res 2003;92:801-8. [Crossref] [PubMed]
- Rochlin DH, Inchauste S, Zelones J, et al. The role of adjunct nanofibrillar collagen scaffold implantation in the surgical management of secondary lymphedema: Review of the literature and summary of initial pilot studies. J Surg Oncol 2020;121:121-8. [PubMed]
- Masià J, Pons G, Rodríguez-Bauzà E. Barcelona Lymphedema Algorithm for Surgical Treatment in Breast Cancer-Related Lymphedema. J Reconstr Microsurg 2016;32:329-35. [Crossref] [PubMed]
- Chang EI, Masià J, Smith ML. Combining Autologous Breast Reconstruction and Vascularized Lymph Node Transfer. Semin Plast Surg 2018;32:36-41. [Crossref] [PubMed]
- Chang EI, Schaverien MV, Hanson SE, et al. Evolution in Surgical Management of Breast Cancer-related Lymphedema: The MD Anderson Cancer Center Experience. Plast Reconstr Surg Glob Open 2020;8:e2674. [Crossref] [PubMed]
- Akita S, Tokumoto H, Yamaji Y, et al. Contribution of Simultaneous Breast Reconstruction by Deep Inferior Epigastric Artery Perforator Flap to the Efficacy of Vascularized Lymph Node Transfer in Patients with Breast Cancer-Related Lymphedema. J Reconstr Microsurg 2017;33:571-8. [Crossref] [PubMed]
- Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015;135:277-85. [Crossref] [PubMed]
- Vibhakar D, Reddy S, Morgan-Hazelwood W, et al. Chimeric pedicled latissimus dorsi flap with lateral thoracic lymph nodes for breast reconstruction and lymphedema treatment in a hypercoagulable patient. Plast Reconstr Surg 2014;134:494e-5e. [Crossref] [PubMed]
Cite this article as: Dionyssiou D, Demiri E. A comprehensive treatment algorithm for patients requiring simultaneous breast and lymphedema reconstruction based on lymph node transfer. Ann Breast Surg 2022;6:33.

