Prophylactic nipple-sparing mastectomy for CHEK2 mutation: a case report
Introduction
Breast cancer is the most common malignancy among women in the western world, with a lifetime incidence commonly cited at 12%. Risk factors include increasing age, early menarche, nulliparity, primigravida after age 30 years, obesity, alcohol abuse, and predisposing genetic mutations. Breast cancer is known to spread insidiously, with up to 40% of patients presenting with regional spread and up to 15% with distant metastasis (1). While Breast Cancer (BRCA) 1/2 is perhaps the most studied breast genetic risk factor, there are other genes that are correlated with increased risk of breast cancer. Checkpoint kinase 2 (CHEK2) is a tumor suppressor gene that encodes CHK2, a serine-threonine kinase that plays a critical role in DNA repair and cell cycle regulation (2).
Mutations in CHEK2 have been implicated in various malignancies. Most notably, the CHEK2*1100delC frameshift mutation confers an elevated risk of breast and colon cancer in both men and women. The frequency of CHEK2*1100delC varies, but seems to be more prevalent in European populations; the 1100delC allele has been identified in up to 2.3–2.9% of Russian, German, and Swedish breast cancer patients compared to 1.1% in the United States (3). A meta-analysis of 26,000 patients with CHEK2*1100delC heterozygosity identified a three- to five-fold increased lifetime risk of breast cancer compared to non-carrier (4). However, mutation penetrance plays a large role in lifetime malignancy risk. The lifetime risk for a woman with both a strong family history of breast cancer and a 1100delC mutation is much higher than that of someone with the same mutation but without a strong family history. The lifetime risk of breast cancer is estimated to be 20% for women with CHEK2 mutations with no affected relative, 28% for women with one affected second-degree relative, 34% for women with one affected first-degree relative, and 44% for women with both a first- and second-degree relative affected (5).
Due to the high likelihood of developing breast cancer (approximately 73% to age 70 years), patients with germline BRCA1/2 mutations may choose to undergo prophylactic mastectomy (1). In high-risk patients under the age of 75, the National Comprehensive Cancer Network (NCCN) recommends individual counseling regarding the decision to undergo prophylactic mastectomy. This counseling should include discussion of the family history, risks, degree of protection, reconstruction options, and alternative options (6). Prophylactic mastectomy has been demonstrated to reduce risk of breast cancer by greater than 90% in women with a moderate to high risk of developing breast cancer (7). However, thus far no studies have demonstrated a clear survival benefit compared to annual monitoring and surgery when breast cancer is detected (8,9).
While traditionally offered to patients with germline BCRA mutations, the NCCN recommendation to discuss the option of prophylactic mastectomy was expanded to include patients with CHEK2*1100delC mutation (6). This change was made in light of aforementioned studies that showed a variable 20–44% lifetime risk of breast cancer for women with CHEK2*1100delC. By pursuing prophylactic breast surgery, patients benefit greatly from decreased risk of future malignancy and potentially reducing anxieties surrounding annual screening. For a patient with CHEK2*1100delC who are also at elevated risk of gastric and renal cancers, surgical reduction of breast cancer risk may improve overall quality of life (10). We present a patient with a family history notable for breast cancers who was found to carry a CHEK2*1100delC mutation that presented to Tripler Army Medical Center in December 2014 to discuss options for prophylactic management. We present the following article in accordance with the CARE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-71/rc).
Case presentation
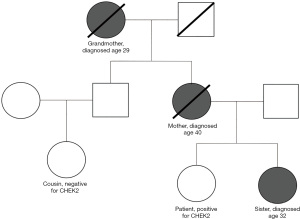
The patient is a 35-year-old nulliparous Caucasian female who initially presented to the general surgery/high-risk breast clinic to discuss cancer risk assessment. She has a strong family history of early onset breast cancer in her mother, grandmother, and sister (Figure 1). Specifically, the patient’s sister was diagnosed with breast cancer at age 32 years and was found to carry a CHEK2*1100delC mutation. The sister underwent bilateral mastectomy and received adjuvant tamoxifen for 10 years. Despite these interventions her disease recurred, presenting with lymph node, bone, and brain metastasis. Her mother was diagnosed with breast cancer at age 40 years with distant metastasis at age 59 years before passing away five years later. The patient’s grandmother had breast cancer and mastectomy performed at age 29 years before passing away from unrelated causes at age 95 years. Genetic testing was not available for the patient’s mother and grandmother. The paternal family history is unremarkable for malignancy. At the time of initial evaluation, the patient was an active-duty officer in the United States Navy which entails frequent prolonged deployments with limited medical resources.
The patient’s past medical history is unremarkable, notably for seasonal allergic rhinitis and left breast biopsy proven fibroadenoma. During her initial breast clinic appointment she denied breast pain, masses, swelling, discharge, skin/nipple changes, retraction, or new lesions. Breast exam was unremarkable. She is a non-smoker and exercises daily while maintaining a healthy body mass index (BMI). Surgical history is remarkable only for bilateral anterior cruciate ligament reconstruction. The patient experienced menarche at age 12 years and has a distant history of brief oral contraceptive use. The patient’s last screening breast magnetic resonance imaging (MRI) was a year prior to clinic presentation which revealed dense fibroglandular breasts bilaterally with no evidence of malignancy. There were no breast masses, architectural distortion, or suspicious eovist enhancement to suggest malignancy. Lymph nodes were scattered throughout both axillae, with no evidence of adenopathy.
Given the patient’s remarkable family history of early onset breast cancers, known CHEK2 mutation in her sister, and its autosomal dominant inheritance pattern, genetic testing was indicated for our patient. Patient’s sister has a known CHEK2 mutation and tested negative for BRCA1/2; therefore, genetic testing was focused primarily on CHEK2. A buccal cell sample was collected and sent for OncoGeneDx® massive parallel sequencing, which revealed a pathogenic heterozygous CHEK2*1100delC mutation. The patient’s lifetime risk of breast cancer was estimated to be 44–50% by a certified genetic counselor, given her two 1st degree relatives (sister and mother) and a 2nd degree relative (grandmother), diagnosed with breast cancer. After reviewing management options, close surveillance, chemoprevention with tamoxifen, or prophylactic breast surgery, she strongly desired bilateral prophylactic mastectomy with emphasis for a good aesthetic outcome. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Treatment
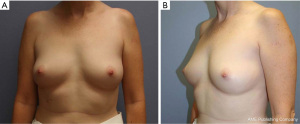
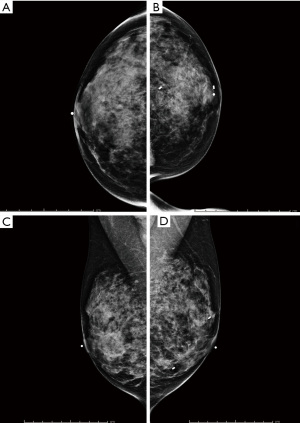
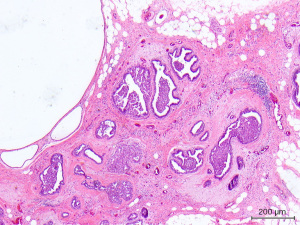
After discussing mastectomy options of total versus subcutaneous (skin vs. nipple-sparing), the patient agreed to undergo prophylactic bilateral nipple-sparing mastectomy with reconstruction. During her pre-op visit, the patient was counseled on the risks of her breast surgery to include nipple ischemia/loss, breast cancer recurrence, skin flap necrosis, wound infection, saline expander rupture/infection, excessive scar formation/pain, poor wound healing, hematoma, and seroma. At this time, pre-operative photos of her breasts were taken (Figure 2). Bilateral pre-operative screening mammography showed Breast Imaging-Reporting and Data System (BI-RADS) Category 2, which was consistent with her previous breast MRI (Figure 3). The patient underwent prophylactic bilateral nipple-sparing mastectomy by inframammary incision with placement of temporary saline tissue expander (Allergan133SV-13-T) with intraoperative fill of 75 mL and Alloderm sling in February 2015. She tolerated the procedure well and was discharged with drains on the 1st post-operative day without complications. The pathology report from the procedure showed benign breast tissue bilaterally, although the right breast was noted to have florid ductal hyperplasia with papillomatosis which is a risk factor for potential malignant transformation (Figure 4).
Outcome and follow-up
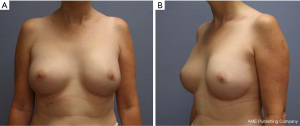
The tissue expander was filled up to 300 mL at an outpatient appointment 5 weeks post-op. It remained in the patient until she underwent bilateral breast implant exchange in June 2015 with an Allergan (Style FF 410) 475 cc anatomic textured full height silicone implant. She returned to surgery clinic for interval follow-up at 6 months after her breast reconstruction and reported satisfaction with the aesthetic result (Figure 5). She was then instructed to follow-up with a clinical breast examination annually, or sooner if she experiences symptoms of implant breakdown such as pain, swelling, contracture, or erythema.
In 2019, the US Food and Drug Administration (FDA) issued a voluntary recall for Allergan textured breast implants and tissue expanders due to increased risk of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). The FDA recommended removal of the Allergan products if patients were having issues with their implants; for asymptomatic patients no revision was recommended due to the low overall incidence of BIA-ALCL (11). The patient was notified of the recall and presented for follow-up appointment in late 2020 to discuss further management. At that point almost six years after her reconstruction, the patient continued to report satisfaction with the cosmetic outcome. She denied breast pain, swelling, discharge, erythema, palpable lump, or contracture. As the patient declined any symptoms concerning for BIA-ALCL, no explantation was indicated according to FDA recommendations. The patient was counseled regarding her breast reconstruction and implants. She was informed that her Allergan implants conferred a greater risk of BIA-ALCL; her overall lifetime risk remains low, on the order of 1:3,345 (12). She was offered an implant exchange with a non-textured silicone implant at the time, but declined. The patient will continue annual clinical breast examinations and will return at the 10-year mark for breast implant revision due to elevated risk of implant rupture and to excise scar contracture if needed. The patient was counseled to seek immediate evaluation from a breast care specialist should she experience symptoms of BIA-ALCL or concerns of implant integrity.
Patient’s perspective
The patient continued a successful career as an officer in the United States Navy. Six years after her procedures, she reports no medical issues with her Allergan breast implants and continues to report a satisfactory cosmetic outcome. Follow-up breast imaging was deferred as patient remained asymptomatic. Per NCCN guidelines, no routine breast imaging is indicated after bilateral prophylactic mastectomy unless there is concern from the patient or palpable abnormality (6).
The knowledge that her risk of CHEK2-related breast cancer remains low is reassuring, particularly since her career entails prolonged deployments with limited medical resources. However, if any symptoms of BIA-ALCL arise while deployed, that would necessitate a medical evacuation (medivac) to a military medical center with surgical capabilities. Fortunately, the overall incidence of BIA-ALCL even in Allergan textured breast implants remains low.
Discussion
To our knowledge, no case reports regarding prophylactic mastectomy for CHEK2 have been published. Due to the elevated lifetime risk of breast cancer in patients with CHEK2*1100delC (20–44%), the NCCN currently recommends individual counseling with consideration of patient preference, individual risk, and reconstruction options. The literature regarding survival benefit from prophylactic bilateral mastectomy for high-risk mutations is mixed. While simulated studies utilizing hypothetical models have predicted survival benefit for high-risk patients, no such conclusions have been derived from actual clinical data to date (13-15).
Although no clear difference in survival has been observed, prophylactic mastectomy may offer psychological benefit through reduced concerns about breast cancer incidence post-operatively. The Mastectomy Reconstruction Outcomes Consortium describes that women who underwent bilateral prophylactic mastectomy report significantly reduced anxiety and improved satisfaction with breast and psychosocial well-being at 1 and 2 years post-surgery (16). This is likely due to the understanding that prophylactic mastectomy reduces the risk of breast cancer in moderate to high-risk patients by more than 90% (7). Bilateral subcutaneous (nipple or skin-sparing) versus total mastectomy may be considered, as both options offer equivalent survival rates (17,18). In the seminal study by Hartmann evaluating risk reduction benefits of prophylactic mastectomy in high-risk patients, no statistically significant difference was identified according to type of mastectomy, total versus subcutaneous (9). Depending on the availability or expertise of plastic surgery consultation, nipple-sparing mastectomy may be the preferred method for an optimal cosmetic outcome despite equivalent rates of recurrence.
Learning points
Patients with the CHEK2*1100delC mutation have up to a 44% lifetime risk of developing breast cancer if they have multiple first-degree family members diagnosed with breast cancer. Prophylactic mastectomy may reduce the risk of developing breast cancer by greater than 90% in high-risk patients. Bilateral nipple-sparing surgery offers similar survival rates to total mastectomy. Because of a lack of evidence suggesting clear survival benefit for prophylactic mastectomy, patients need to carefully consider risks, degree of protection, reconstruction options, and alternative options. For patients who have undergone prophylactic mastectomy and reconstruction with Allergan textured breast implants or tissue expanders, they are at elevated risk for Breast Implant Associated Anaplastic Large Cell Lymphoma. These implants should be removed if patients experience persistent pain, swelling, palpable lump, contracture, axillar lymphadenopathy, or B-type symptoms. No explantation is recommended for patients that are asymptomatic, due to overall low incidence of BIA-ALCL.
Acknowledgments
The authors would like to thank Dr. Judy Aeum, Anatomical Pathology Chief at Tripler Army Medical Center, for providing images of the patient’s histopathological specimens. The authors would also like to thank Ms. Susan Donlon, certified genetic counselor, for the genetic counseling and risk assessment for this patient.
Funding: This work was supported by the General Surgery Service at Tripler Army Medical Center.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-71/rc
Disclaimer: The views expressed in this manuscript are those of the authors alone, and do not reflect the opinions of the United States Government, Department of Defense or the United States Army.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-71/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vogel VG. Breast cancer prevention: a review of current evidence. CA Cancer J Clin 2000;50:156-70. [Crossref] [PubMed]
- Desrichard A, Bidet Y, Uhrhammer N, et al. CHEK2 contribution to hereditary breast cancer in non-BRCA families. Breast Cancer Res 2011;13:R119. [Crossref] [PubMed]
- Apostolou P, Papasotiriou I. Current perspectives on CHEK2 mutations in breast cancer. Breast Cancer (Dove Med Press) 2017;9:331-5. [Crossref] [PubMed]
- Weischer M, Bojesen SE, Ellervik C, et al. CHEK2*1100delC genotyping for clinical assessment of breast cancer risk: meta-analyses of 26,000 patient cases and 27,000 controls. J Clin Oncol 2008;26:542-8. [Crossref] [PubMed]
- Cybulski C, Wokołorczyk D, Jakubowska A, et al. Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol 2011;29:3747-52. [Crossref] [PubMed]
- Daly MB, Pal T, Berry MP, et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:77-102. [Crossref] [PubMed]
- Guillem JG, Wood WC, Moley JF, et al. ASCO/SSO review of current role of risk-reducing surgery in common hereditary cancer syndromes. J Clin Oncol 2006;24:4642-60. [Crossref] [PubMed]
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004;22:1055-62. [Crossref] [PubMed]
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999;340:77-84. [Crossref] [PubMed]
- Näslund-Koch C, Nordestgaard BG, Bojesen SE. Increased Risk for Other Cancers in Addition to Breast Cancer for CHEK2*1100delC Heterozygotes Estimated From the Copenhagen General Population Study. J Clin Oncol 2016;34:1208-16. [Crossref] [PubMed]
- The FDA Requests Allergan Voluntarily Recall Natrelle BIOCELL Textured Breast Implants and Tissue Expanders from the Market to Protect Patients: FDA Safety Communication. FDA 2020. Available online: https://www.fda.gov/medical-devices/safety-communications/fda-requests-allergan-voluntarily-recall-natrelle-biocell-textured-breast-implants-and-tissue
- Parham CS, Hanson SE, Butler CE, et al. Advising patients about breast implant associated anaplastic large cell lymphoma. Gland Surg 2021;10:417-29. [Crossref] [PubMed]
- Giannakeas V, Narod SA. The expected benefit of preventive mastectomy on breast cancer incidence and mortality in BRCA mutation carriers, by age at mastectomy. Breast Cancer Res Treat 2018;167:263-7. [Crossref] [PubMed]
- Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surg 2016;212:660-9. [Crossref] [PubMed]
- Kurian AW, Lichtensztajn DY, Keegan TH, et al. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998-2011. JAMA 2014;312:902-14. [Crossref] [PubMed]
- Carlson GW, Bostwick J 3rd, Styblo TM, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg 1997;225:570-5; discussion 575-8. [Crossref] [PubMed]
- Seki T, Jinno H, Okabayashi K, et al. Comparison of oncological safety between nipple sparing mastectomy and total mastectomy using propensity score matching. Ann R Coll Surg Engl 2015;97:291-7. [Crossref] [PubMed]
- Lewis RS, George A, Rusby JE. Nipple-sparing mastectomy in women at high risk of developing breast cancer. Gland Surg 2018;7:325-36. [Crossref] [PubMed]
Cite this article as: Yuan K, Lin-Hurtubise KM, Lee MY. Prophylactic nipple-sparing mastectomy for CHEK2 mutation: a case report. Ann Breast Surg 2022;6:40.