
Standards in oncoplastic breast-conserving surgery
Introduction
Oncoplastic breast-conserving surgery refers to the surgical management of breast cancer which combines oncologic techniques for partial mastectomy with plastic surgical techniques to optimize breast aesthetics and symmetry. The goals of oncoplastic breast-conserving surgery therefore include (I) oncologic efficacy comparable to partial mastectomy alone, (II) improved breast aesthetics and symmetry compared to partial mastectomy alone, (III) a favorable safety profile regarding complications and need for re-operation, and (IV) improved overall patient satisfaction compared to partial mastectomy alone.
Oncoplastic techniques have increased in popularity over time with greater acceptance of their effectiveness and safety profile, as well as greater surgeon comfort with the technical aspects of the operations. A recent retrospective cohort analysis of data from the ACS-NSQIP database demonstrated an increase in use of oncoplastic breast reconstruction of 241%, a rate of increase of 11% per year, while the rate of partial mastectomy without reconstruction remained relatively constant (1). Oncoplastic breast reconstruction is now considered by many to be the “gold standard” following partial mastectomy (2,3). However, there remains disagreement among experts regarding several aspects of oncoplastic reconstruction including the nomenclature used to describe, classify and bill for oncoplastic surgical procedures (4), the importance of dedicated training programs in oncoplastic surgery (5,6), and the necessity of plastic surgeon involvement in oncoplastic reconstruction cases (7). In many cases, points of view vary significantly by geographical location. All stakeholders recognize the urgent need for standardization of these items in order to improve communication between breast and plastic surgeons worldwide, to facilitate data sharing and generalizability, and most importantly to improve patient outcomes. In this review, we aim to summarize current standards as they pertain to oncoplastic terminology, techniques, and safety.
Standard terminology in oncoplastic surgery
In April 2019, to improve consistency and minimize confusion among patients and surgeons, the American Society of Breast Surgeons (ASBrS) published a consensus definition of oncoplastic surgery as, “a form of breast-conservation surgery that includes oncologic resection with a partial mastectomy, ipsilateral reconstruction using volume displacement or volume replacement techniques with possible contralateral symmetry surgery when appropriate” (8). Regional differences in the acceptance of this definition may exist; though some surgeons may consider oncoplastic surgery to include any method of breast reconstruction after partial or total mastectomy (9,10), others (particularly in the United States) use the terms “oncoplastic surgery” and “oncoplastic breast conservation” interchangeably. For the remainder of this paper, the term “oncoplastic surgery” will refer specifically to methods of breast reconstruction after partial mastectomy.
Fundamentally, oncoplastic surgery involves tumor removal, preservation of breast tissue and reconstruction of the defect. The oncoplastic approach was pioneered by Audretsch et al. as a way of addressing not only the oncologic resection but also as a way of reconstructing the breast to a reasonable form (11,12). This intent was further reinforced by Clough et al. whose classification system heavily influenced the ASBrS definition (13). This has been supported by both breast and plastic surgeons (14-18), and oncoplastic surgery has becomes a third standard of surgery offered to breast cancer patients. Along with the previous two traditional options of standard partial mastectomy and mastectomy, oncoplastic surgery is now a third option for the appropriate breast cancer patient.
Classification systems
Most classifications differentiate oncoplastic surgery into volume displacement and volume replacement techniques (8,19,20). A level 1 volume displacement oncoplastic operation involved less than 20% of the breast tissue being removed in the partial mastectomy and then reconstructed with a local tissue rearrangement design such as a doughnut mastopexy or a crescent mastopexy (8). A Level 2 volume displacement oncoplastic operation involved 20% to 50% of the breast tissue being removed in the partial mastectomy followed by a reconstruction design that typically uses breast mastopexy or reduction designs (see Figures 1-4). Lastly, a volume replacement oncoplastic operation occurs when greater than 50% of breast tissue is removed as part of the partial mastectomy followed by reconstruction using local/regional flaps or implants. The ASBrS classification is meant as a guide; however, the final surgical plan is always made as a shared decision between the patient and the recommendations of the surgical team. Selection of operation depends on the oncologic features of the breast cancer as well as the patient’s pre-morbid breast appearance; these features are balanced against the patient’s preferences and expectations. For example, a patient with a small breast cancer in the inferior pole, moderate sized breasts and Grade 3 ptosis may benefit from an oncoplastic mastopexy design even with the possibility that less than 20% of the breast tissue may be removed as part of the partial mastectomy; selection of a mastopexy reconstructive design in this scenario would prevent the development of a bird beak deformity (21). Nevertheless, the majority of oncoplastic operations may be able to use this classification system as a useful algorithm for guiding selection of surgical technique.
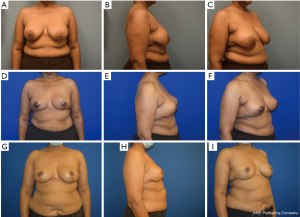
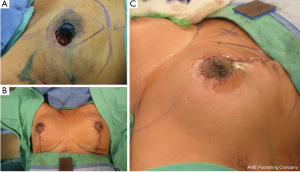
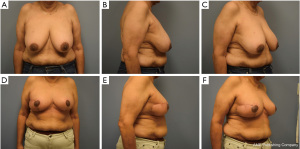
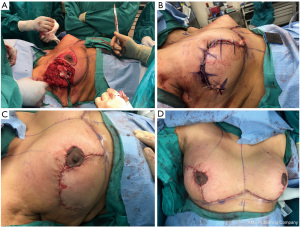
Multidisciplinary team approach
A multidisciplinary approach to the care of patients undergoing oncoplastic procedures is essential, as in the care of any patient with breast cancer. Multimodal therapies and an individualized approach to treatment will mandate coordination of care between team members from radiology, surgical oncology, hematology, radiation oncology, pathology, and others. Communication between these teams in the preparation/planning stages of treatment in a multidisciplinary tumor board setting is a widely recognized standard of care, with the overarching goal of achieving the best possible oncologic outcome while maintaining the best possible breast aesthetic. Though final breast cosmesis is obviously important in overall patient satisfaction, opinions among specialists regarding the necessity of plastic surgeon involvement in oncoplastic procedures vary significantly. In some specialized breast surgery practices, a general or oncologic surgeon performs both the ablative and reconstructive portions of oncoplastic surgeries. In other institutions, a two-team approach with ablation performed by surgical oncology followed by reconstruction by plastic surgery is the accepted standard.
Preference for and opinions regarding the necessity of a single team versus a two-team approach vary depending on specialty, training experience, and geographic location. For example, in the United States, a two-team approach has traditionally been employed (22). Many breast surgeons feel comfortable performing Level 1 volume displacement local tissue rearrangements after smaller partial mastectomy operations, and the importance of hidden incisions and aesthetics is now being taught in the breast surgery curriculum. The majority of breast surgeons presently do not perform Level 2 volume displacement oncoplastic surgery themselves and require the partnership of a plastic surgeon to safely perform such operations.
A recent survey of members of the American Society of Breast Surgeons and the American Society of Plastic Surgery was performed to ascertain differences in opinion regarding partial breast reconstruction at the time of tumor resection between breast surgeons and plastic surgeons (7). This survey found that while plastic surgeons were more likely to favor a two-team approach overall, the preference for either two-team approach or a mutually agreed upon team combination was favored by both breast and plastic surgeons, and only 7.5% of respondents felt that it was appropriate for a breast surgeon alone to perform more complex reconstructions. Plastic surgeon availability was not felt to be a major barrier to partial breast reconstruction by either group. A subsequent American Society of Breast Surgeons survey in the following years noted that 99% of breast surgeons surveyed were interested in oncoplastic surgery and approximately 19% of those had independently performed a Level 2 volume displacement oncoplastic operation using a mastopexy/reduction design (23). Regardless of the single surgeon versus two-team approach, such interests underscore the need for further oncoplastic surgery adoption with particular emphasis on safety and appropriate training.
The single surgeon model has been popular in the UK and in parts of Europe and now, thanks to “dual training” opportunities, is used in the US as well. A recent survey in the United Kingdom regarding changing practice patterns in oncoplastic surgeries suggested a threefold decrease in oncoplastic procedures performed using a two-team approach (5). The proportion of general and breast surgeons in the UK who performed breast mastopexy and reduction procedures increased by 26%, and the proportion who performed latissimus dorsi flaps increased by 15% between 2010 and 2015. The authors of this study theorized that fewer plastic surgeons and high cross-specialty demand limited plastic surgery availability and participation in oncoplastic procedures.
A practice survey of general surgeons in Ontario, Canada found that less than 50% of respondents performed oncoplastic procedures, and that most commonly, plastic surgeons were involved in breast conserving surgeries rarely (44.0% of respondents) or never (44.6% of respondents) (6). Lack of specific training in oncoplastic techniques and lack of plastic surgeon availability were cited as the major barriers to more widespread adoption.
In the only study to compare oncoplastic surgical outcomes following a single team versus two-team approach, Blankensteijn et al. retrospectively evaluated the NSQIP database for patients undergoing oncoplastic reconstruction between 2005 and 2017 (24). A total of 4,350 patients met criteria; of these, 3,759 had undergone oncoplastic reconstruction by a breast surgeon alone, and 591 by a plastic surgeon and breast surgeon together. There was no significant difference in the rate of post-operative complications between the two groups, though the authors found that plastic surgery involvement likely correlated with more complex reconstructive procedures. The authors concluded that neither a single or two-team approach was associated with increased surgical morbidity. However, it should be noted that the majority of single-surgeon oncoplastic surgeries performed were Level 1 volume displacement with less complex techniques compared to oncoplastic operations utilizing the two-surgeon model that used a greater proportion of Level 2 volume displacement techniques.
Regardless of surgical specialty or country of origin, all parties can agree that achieving the best possible aesthetic breast appearance is in the best interest of the patient. In practice settings where plastic surgeons are readily available, a two-team approach makes sense and has several advantages. In situations where ability to coordinate with plastic surgery is limited, patients should not have to settle for a lower standard of care. In these situations, additional training for general or breast surgeons in advanced oncoplastic techniques in order to deliver a high quality oncologic and reconstructive procedure should be the goal.
Training in oncoplastic surgery
To ensure acceptance and patient safety, training is critically important when it comes to oncoplastic surgery regardless of which model is utilized.
The “single-surgeon model” implies that the treating surgeon has expertise in both the oncologic treatment of breast cancer as well as reconstruction of partial mastectomy defects. In the United States, there are different options for obtaining training and expertise in these areas. One potential track involves training in an integrated plastic surgery program followed by a one-year American Society of Breast Surgeons/Society of Surgical Oncology approved breast surgery fellowship. Another option involves general surgery residency followed by formal plastic surgery training and then a one-year American Society of Breast Surgeons/Society of Surgical Oncology approved breast surgery fellowship. It is expected that plastic surgery training in the United States would involve exposure to oncoplastic breast reconstruction. Potential options in the future to shorten training would be a 2–3 year oncologic and reconstructive breast oncoplastic training fellowship after general surgery. Collaboration between the major surgical societies is key to success of these programs.
The “two-surgeon model” requires that both breast surgeon and plastic surgeon understand the nuances of oncoplastic surgery. Training is acquired in a formal setting through surgical residency programs in either breast or plastic surgery, or through societal training courses.
Outside of the United States, other countries have developed oncoplastic training opportunities that uniquely suit their particular needs and resources. Given that some of the first adopters of oncoplastic surgery came from Europe, it is not surprising that formal oncoplastic training programs have since been developed in European countries. For example, in Britain, the Joint Committee on Surgical Training has established a formal oncoplastic breast surgery fellowship that is overseen by the Association of Breast Surgery and the British Association of Plastic, Reconstructive and Aesthetic Surgeons. Applicants for this fellowship can come from both general surgery or plastics surgery training backgrounds (25). In Australia and New Zealand, the Breast Surgeons of Australia and New Zealand (BreastSurgANZ) have developed a two-year post fellowship training program that formally trains breast fellows in both Level 1 and Level 2 volume displacement oncoplastic surgery (26). In Brazil, specialized oncoplastic training centers have developed specialized courses where practicing surgeons with backgrounds in either oncology, breast surgery or plastic surgery can apply to learn new skill sets required to be safe in practicing oncoplastic techniques (27).
Safety of oncoplastic surgery
Oncoplastic surgery aims to optimize the final cosmetic appearance of the breast following partial mastectomy; however, breast aesthetics are secondary in importance to oncologic efficacy and safety. Many oncoplastic techniques involve extensive rearrangement of local tissues, creation of additional incisions on the breast, or transposition of regional tissues into the tumor cavity. Legitimate concerns have been previously raised about how these techniques may affect overall risk of complications, subsequent delivery of adjuvant therapy, margin positivity, local recurrence, and survival. Preoperative counseling of patients considering oncoplastic breast surgery should include a thorough discussion of the risks and benefits of these techniques. Surgeons who perform or participate in oncoplastic surgeries should have a shared understanding and agreement about the safety profile of these procedures.
Surgical complications following oncoplastic breast reconstruction
Oncoplastic breast reconstruction can be directly compared to alternative therapies including standard breast conservation without reconstruction, as well as total mastectomy with or without reconstruction. In primarily retrospective analyses, these comparisons have been performed demonstrating a favorable risk profile for oncoplastic techniques. The overall complication rate following oncoplastic surgery ranges from 14–16% in systematic review and meta-analysis of the literature (28,29). Common complications include delayed wound healing, fat necrosis, infection, nipple necrosis, seroma and hematoma, with individual incidence ranging from <1–4% (28,30). Overall, the rate of complication requiring reoperation is likely around 3% (29,31). In their NSQIP database analysis, Cil et al. identified multiple factors independently associated with a higher likelihood of developing a complication within 30 days of surgery including obesity, smoking, American Academy of Anesthesiologists (ASA) category 3 or 4, diabetes, bleeding disorder, chronic obstructive pulmonary disease (COPD), and a longer operative time. The presence of bleeding disorder had the highest association with post-operative complications (odds ratio 1.8) (32).
Smoking in patients undergoing breast reconstruction increases perioperative morbidity and mortality as well as cost to the healthcare system (33). Smoking in patients undergoing oncoplastic breast reconstruction is a situation that demands special consideration; though smoking increases the risk of post-operative complications, the risk of attempting reconstruction on a radiated breast in a delayed setting may be even greater (34). Therefore, patients with smoking history must be counseled regarding the risks and benefits of undergoing immediate oncoplastic reconstruction for their ipsilateral (cancer) breast. Smoking remains an important modifiable risk factor, and cessation counseling is appropriate. If needed, the contralateral symmetry operations can be delayed until the patient stops smoking, providing yet another incentive for smoking cessation.
Overall, oncoplastic reconstruction may have a comparable or slightly lower rate of complications compared to standard breast conservation therapy alone. A meta-analysis performed by Losken et al. demonstrated a rate of complications of 15.5% in patients undergoing oncoplastic reconstruction, compared to 25.9% in patients undergoing standard breast conservation therapy, though the average follow-up of patients in this analysis was longer for patients undergoing breast conservation alone (64 vs. 37 months) (29). In their NSQIP review, Cil et al. found that the 30-day rate of complications was similar between patients undergoing oncoplastic reconstruction (1.7%) vs. standard breast conservation therapy (1.9%) (32).
When complications do occur, significant delay in initiation of adjuvant therapy is possible. Kapadia et al. retrospectively reviewed 118 patients who underwent oncoplastic reconstruction at a single institution (35). Twenty-two percent of patients developed a complication including delayed wound healing, seroma, infection, and wound dehiscence. There was a statistically significant delay in initiation of radiation in patients who developed a complication versus those who did not (74 vs. 54 days, P<0.001). Similarly, in a retrospective review of 150 patients undergoing oncoplastic reconstruction published by Hillberg et al., initiation of adjuvant radiotherapy was delayed in 8.2% of patients due to a post-operative complication, though the overall complication rate was high in this study (37.5%) (36).
Breast reduction or mastopexy is often considered for the contralateral or non-cancer breast in order to improve breast symmetry and optimize aesthetic appearance following oncoplastic reconstruction. Concerns have been raised that this additional surgery may increase the rate of post-operative complications and potentially delay adjuvant therapy. In a recent retrospective review published by Deigni et al., 429 patients underwent oncoplastic reconstruction followed by either immediate contralateral symmetry procedure, or symmetry procedure performed in a delayed fashion (37). There was no significant difference in overall complications between the two groups. Though complications resulted in a delay in adjuvant therapy in 4.2% of patients overall, complications attributable to the contralateral symmetry procedure accounted for a delay in only 0.7% of patients.
Surgical margins following oncoplastic reconstruction
Positive margins following breast conservation are known to correlate with cancer recurrence. One theoretical benefit of oncoplastic reconstruction compared to standard breast conservation is that the enhanced ability to aesthetically reconstruct large breast defects may encourage the extirpative surgeon to perform more generous tumor resections, resulting in lower rates of positive margins. In a retrospective review by Losken et al. of 207 patients undergoing breast conservation, positive margin rates were compared in patients who had lumpectomy followed by oncoplastic reconstruction versus those who had lumpectomy alone (38). The authors found that patients undergoing oncoplastic reconstruction had significantly lower positive margin rates (defined as <1 mm), lower rates of re-excision, and lower completion mastectomy rates compared to lumpectomy alone despite more advanced cancers in the oncoplastic group. This finding was confirmed in a meta-analysis of more than 8,500 patients performed by the same group; the overall rate of margin positivity was 12% in the oncoplastic group compared to 21% in patients undergoing standard breast conservation (29). A similar systematic literature review in early stage breast cancer patients reinforced a low positive margin rate in oncoplastic surgery of 10% by De La Cruz et al. (28). Invasive lobular tumor histology, ductal carcinoma in-situ tumor histology, obesity, tumor multifocality and presence of microcalcifications on mammogram have been shown to predict margin positivity and need for re-excision following oncoplastic surgery (39-41).
Local recurrence, disease free and overall survival
To be considered a safe surgical option for patients with breast cancer, oncoplastic techniques must not sacrifice the oncologic efficacy achievable with standard breast conservation or mastectomy. Given that oncoplastic breast reconstruction techniques have only become a mainstream treatment option in the last 2 decades, long term data about recurrence and survival are somewhat lacking. As previously mentioned, margin positivity following partial mastectomy is known to predict local recurrence; however, tumor biology is also an important predictor of oncologic outcome. Oncoplastic surgical techniques extend the indications for breast conservation, including patients with larger and more aggressive tumors. Concerns have been raised that this phenomenon may affect the rate of cancer recurrence in patients treated with oncoplastic techniques. In a retrospective cohort study of 1,800 patients with breast cancer who underwent either standard breast conservation or oncoplastic breast conservation, Niinikoski et al. addressed this question (42). After a median follow-up of 75 months, there was no difference in local recurrence-free survival between the two groups. Of particular note, patients treated in the oncoplastic group had significantly larger tumors which were more often palpable and multifocal; in addition, their breast cancers had significantly higher histologic grade, T-stage and lymph node involvement. There was no difference in positive margin rate between groups in this study.
In a systematic review performed in 2016, De La Cruz et al. analyzed 6,011 oncoplastic reconstruction patients with a mean follow-up of 50.5 months. Among 871 patients with at least 5 years follow-up, the rates of overall survival, disease-free survival, local recurrence and distant recurrence were 93.4%, 85.4%, 6% and 11.9% respectively (28). The authors noted that these rates appear to correlate favorably with recurrence and survival rates after standard breast conservation, suggesting that surgical technique is not the primary predictor of oncologic outcome.
In general, it appears that oncologic reconstruction techniques do result in a generous resection and improved margin control, however, this does not translate into a recurrence benefit compared to standard breast conservation. Tumor recurrence, however, is not increased by the immediate reconstruction of these defects. Oncoplastic surgery may be offered to patients with a broader range of tumor size and pathology, and the aesthetic benefits of this approach do not appear to compromise cancer recurrence and survival.
Patient satisfaction following oncoplastic surgery
The primary perceived advantage of oncoplastic surgery is the aesthetic improvement in the final breast appearance compared to standard breast conservation, in which the rate of unacceptable breast cosmesis may be as high as 40% (43). Though oncoplastic surgeries have in common reconstruction of a partial mastectomy defect, the techniques by which this is accomplished and the oncologic situation in which they are applied may significantly affect how patients perceive benefit following surgery. For example, a patient who undergoes volume displacement/mastopexy for reconstruction of a relatively small partial breast defect will likely have a different experience than a patient who undergoes volume replacement with autologous tissue reconstruction of a large defect followed by adjuvant therapy for locally advanced disease. Treatment of the contralateral breast may also have a large impact on patient satisfaction, as breast symmetry is highly correlated with overall cosmesis. An analysis of patient reported outcomes after oncoplastic surgery using standardized, validated questionnaires will inform patient counseling and surgical decision making in the pre-operative setting.
Patient satisfaction following oncoplastic reconstruction has been shown to exceed satisfaction following standard breast conservation therapy (44,45), mastectomy alone (46), and mastectomy with reconstruction (47-49). Veiga et al. compared the patient reported satisfaction scores from 45 women undergoing breast conservation and oncoplastic reconstruction with 42 women who underwent breast conservation alone using validated questionnaires (45). He found that patients who underwent oncoplastic reconstruction reported higher levels of perceived health and physical functioning, higher social-emotional well-being and self-esteem compared to the standard breast conservation group. In addition, he noted that patients in the oncoplastic reconstruction group actually had improvement in their satisfaction scores in follow-up compared to before surgery. Rose et al. published the results of a survey study comparing patient reported outcomes after oncoplastic surgery (107 patients) or standard breast conservation (657 patients) (44). Subjects were administered the Breast-Q validated questionnaire an average of 60.8 months from the time of surgery. The authors found that despite having on average more advanced cancers, patients in the oncoplastic group had significantly higher self-reported psychosocial well-being. A comprehensive literature review of patient reported outcome measures including Breast-Q was performed by Char et al. and found that oncoplastic surgery in general had the highest patient satisfaction scores among breast reconstructive choices (49). Forty three articles were included in this study looking at all forms of autologous tissue and implant based reconstruction.
The method of oncoplastic reconstruction or extent of surgery seems to have little impact on patient satisfaction. High levels of patient satisfaction have been reported after volume displacement techniques (46,50) as well as volume replacement techniques (51,52). In their survey of 624 patients undergoing a variety of different oncoplastic procedures, Rezai et al. demonstrated that there was no significant correlation between the method of oncologic reconstruction and the patient perception of the aesthetic result. Oncoplastic reconstruction with a reduction mammaplasty approach may have a particularly large impact on patient-reported quality of life after surgery. Losken et al. performed a retrospective review of 353 patients undergoing oncoplastic breast reconstruction with a breast reduction technique (53). The average reduction weight of patients in this study was 545 g. The authors used the Breast-Q validated questionnaire to show that, compared to pre-operative baseline, women undergoing oncoplastic reduction had increased self-confidence, feelings of attractiveness, emotional health and satisfaction with sex life over 1 year post-operatively.
There is some evidence that suggests that oncologic status may affect patient reported outcomes more than surgical technique. In their study of 120 patients undergoing oncoplastic breast reconstruction with volume displacement techniques, Gardfjell et al. showed that lower patient satisfaction appeared to correlate with need for axillary dissection and neoadjuvant chemotherapy (50). In their comparison of 379 patients undergoing oncoplastic surgery or breast conservation alone, Ojala et al. showed that larger tumor diameter, multifocality, and oncoplastic reconstruction were predictive of poor patient-reported aesthetic result; however, in this study, patients undergoing oncoplastic reconstruction were more likely to have larger, multifocal tumors with lymph node involvement (54).
Taken together, it can be said that patients undergoing oncoplastic reconstruction have high levels of satisfaction with their appearance, mental well-being, and overall perception of health, comparing favorably to other surgical breast cancer treatment modalities. This effect is somewhat expected and may be secondary to the attention to breast aesthetics and symmetry that are the focus of oncoplastic techniques. The quality-of-life benefit that accompanies breast reduction may also be a contributing factor. This data can assist with patient counseling and decision making.
Conclusions
Oncoplastic breast reconstruction is now a globally accepted option for treatment of breast cancer. This approach has a favorable safety profile and equivalent oncologic efficacy compared to standard breast conservation, but has the major advantage of improved aesthetic outcomes. By utilizing techniques of volume displacement, volume replacement, and contralateral breast reduction/mastopexy, the oncoplastic approach can reduce the rate of post-lumpectomy breast deformity while optimizing breast symmetry.
The oncoplastic approach mandates multidisciplinary communication and coordination in order to provide the highest quality care for patients. Inter-specialty discussion in the pre-operative planning phase, particularly between surgical oncology and plastic surgery, will optimize the plan of care from both oncologic and reconstructive standpoints.
When considering the delivery of oncoplastic reconstructive care from a global viewpoint, one size does not fit all. Breast surgeon comfort with oncoplastic techniques and the involvement of a plastic surgeon in oncoplastic operations may vary significantly by geographic location depending on availability of training and subspecialty resources. While there is no accepted standard for who should be performing oncoplastic surgery, the goal should be that all involved have appropriate training and education in order to deliver the highest possible quality of patient care.
Currently available data suggest excellent outcomes in oncologic efficacy, aesthetic result, overall safety and patient satisfaction. Previously limited given the relative novelty of oncoplastic techniques, data quality is improving with larger series and longer follow-up.
As international acceptance of oncoplastic reconstruction continues to increase, providers should continue to evaluate outcomes, refine techniques, and streamline care delivery through better interspecialty communication with the goal of optimizing results and overall patient satisfaction.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard and Jørn Bo Thomsen) for the series “Breast Reconstruction—The True Multidisciplinary Approach” published in Annals of Breast Surgery. The article has undergone external peer review.
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-33/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: https://abs.amegroups.com/article/view/10.21037/abs-21-33/coif). The series “Breast Reconstruction—The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. AC and AL report consulting fees related to implants which have no impact on this manuscript. AC serves as an unpaid editorial board member of Annals of Breast Surgery from March 2021 to February 2023. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jonczyk MM, Jean J, Graham R, et al. Surgical trends in breast cancer: a rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res Treat 2019;173:267-74. [Crossref] [PubMed]
- Hernanz F, González-Noriega M, Sánchez S, et al. Oncoplastic breast conserving surgery with tailored needle-guided excision. Gland Surg 2017;6:698-705. [Crossref] [PubMed]
- Macmillan RD, McCulley SJ. Oncoplastic Breast Surgery: What, When and for Whom? Curr Breast Cancer Rep 2016;8:112-7. [Crossref] [PubMed]
- Weber WP, Soysal SD, El-Tamer M, et al. First international consensus conference on standardization of oncoplastic breast conserving surgery. Breast Cancer Res Treat 2017;165:139-49. [Crossref] [PubMed]
- Challoner T, Skillman J, Wallis K, et al. Oncoplastic techniques: Attitudes and changing practice amongst breast and plastic surgeons in Great Britain. Breast 2017;34:58-64. [Crossref] [PubMed]
- Maxwell J, Roberts A, Cil T, et al. Current Practices and Barriers to the Integration of Oncoplastic Breast Surgery: A Canadian Perspective. Ann Surg Oncol 2016;23:3259-65. [Crossref] [PubMed]
- Losken A, Kapadia S, Egro FM, et al. Current Opinion on the Oncoplastic Approach in the USA. Breast J 2016;22:437-41. [Crossref] [PubMed]
- Chatterjee A, Gass J, Patel K, et al. A Consensus Definition and Classification System of Oncoplastic Surgery Developed by the American Society of Breast Surgeons. Ann Surg Oncol 2019;26:3436-44. [Crossref] [PubMed]
- Radtke C. Standards in Oncoplastic Breast Reconstruction. Breast Care (Basel) 2019;14:269-70. [Crossref] [PubMed]
- Weber WP, Haug M, Kurzeder C, et al. Oncoplastic Breast Consortium consensus conference on nipple-sparing mastectomy. Breast Cancer Res Treat 2018;172:523-37. [Crossref] [PubMed]
- Chatterjee A. Author's Response to Reflexion on Consensus Statement on Oncoplastic Surgery, by Zucca-Matthes, Gustavo, et al. Ann Surg Oncol 2019;26:3007-8. [Crossref] [PubMed]
- Audretsch WP, Rezai M, Kolotas C, et al. Tumor-specific immediate reconstruction in breast cancer patients. Perspectives in Plastic Surgery 1998;11:71-100.
- Clough KB, Ihrai T, Oden S, et al. Oncoplastic surgery for breast cancer based on tumour location and a quadrant-per-quadrant atlas. Br J Surg 2012;99:1389-95. [Crossref] [PubMed]
- Clough KB, Meredith I. The Oncoplastic Frenzy: Beware the Swing of the Pendulum. Ann Surg Oncol 2019;26:3792-3. [Crossref] [PubMed]
- Silverstein MJ. Oncoplastic Breast Surgery: From Oblivion to Mainstream. Ann Surg Oncol 2019;26:3409-12. [Crossref] [PubMed]
- Losken A, Hart AM, Chatterjee A. Updated Evidence on the Oncoplastic Approach to Breast Conservation Therapy. Plast Reconstr Surg 2017;140:14S-22S. [Crossref] [PubMed]
- Losken A, Chatterjee A. Improving Results in Oncoplastic Surgery. Plast Reconstr Surg 2021;147:123e-34e. [Crossref] [PubMed]
- Molina BJ, Shelby RD, Janis JE. Key Areas for Development in Oncoplastic Breast Reconstruction. Plast Reconstr Surg Glob Open 2020;8:e3273. [Crossref] [PubMed]
- Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375-91. [Crossref] [PubMed]
- Margenthaler JA, Dietz JR, Chatterjee A. The Landmark Series: Breast Conservation Trials (including oncoplastic breast surgery). Ann Surg Oncol 2021;28:2120-7. [Crossref] [PubMed]
- Patel K, Bloom J, Nardello S, et al. An Oncoplastic Surgery Primer: Common Indications, Techniques, and Complications in Level 1 and 2 Volume Displacement Oncoplastic Surgery. Ann Surg Oncol 2019;26:3063-70. [Crossref] [PubMed]
- Losken A, Nahabedian MY. Oncoplastic breast surgery: past, present, and future directions in the United States. Plast Reconstr Surg 2009;124:969-72. [Crossref] [PubMed]
- Chatterjee A, Gass J, Burke MB, et al. Results from the American Society of Breast Surgeons Oncoplastic Surgery Committee 2017 Survey: Current Practice and Future Directions. Ann Surg Oncol 2018;25:2790-4. [Crossref] [PubMed]
- Blankensteijn LL, Crystal DT, Egeler SA, et al. The Influence of Surgical Specialty on Oncoplastic Breast Reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2248. [Crossref] [PubMed]
- Liem AA, Iqbal A. Oncoplastic breast surgery in Britain. Plast Reconstr Surg 2011;127:1012-3. [Crossref] [PubMed]
- Yunaev M, Hingston G. Oncoplastic breast surgery in Australia and New Zealand-2014 and beyond. Gland Surg 2014;3:77-80. [PubMed]
- Pires DdM, Gazoto Junior O, Valadares CN, et al. Training in oncoplastic and reconstructive breast surgery: analysis of training in America and in the European Union with the brazilian reality. Mastology (Impr) 2017:164-71.
- De La Cruz L, Blankenship SA, Chatterjee A, et al. Outcomes After Oncoplastic Breast-Conserving Surgery in Breast Cancer Patients: A Systematic Literature Review. Ann Surg Oncol 2016;23:3247-58. [Crossref] [PubMed]
- Losken A, Dugal CS, Styblo TM, et al. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014;72:145-9. [Crossref] [PubMed]
- Piper ML, Esserman LJ, Sbitany H, et al. Outcomes Following Oncoplastic Reduction Mammoplasty: A Systematic Review. Ann Plast Surg 2016;76:S222-6. [Crossref] [PubMed]
- Fitoussi AD, Berry MG, Famà F, et al. Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases outcomes article. Plast Reconstr Surg 2010;125:454-62. [Crossref] [PubMed]
- Cil TD, Cordeiro E. Complications of Oncoplastic Breast Surgery Involving Soft Tissue Transfer Versus Breast-Conserving Surgery: An Analysis of the NSQIP Database. Ann Surg Oncol 2016;23:3266-71. [Crossref] [PubMed]
- Bloom JA, Rashad R, Chatterjee A. The Impact on Mortality and Societal Costs From Smoking Cessation in Aesthetic Plastic Surgery in the United States. Aesthet Surg J 2019;39:439-44. [Crossref] [PubMed]
- Bloom JA, Asban A, Tian T, et al. A Cost-Utility Analysis Comparing Immediate Oncoplastic Surgery with Delayed Oncoplastic Surgery in Smoking Breast Cancer Patients. Ann Surg Oncol 2021;28:2579-88. [Crossref] [PubMed]
- Kapadia SM, Reitz A, Hart A, et al. Time to Radiation After Oncoplastic Reduction. Ann Plast Surg 2019;82:15-8. [Crossref] [PubMed]
- Hillberg NS, Meesters-Caberg MAJ, Beugels J, et al. Delay of adjuvant radiotherapy due to postoperative complications after oncoplastic breast conserving surgery. Breast 2018;39:110-6. [Crossref] [PubMed]
- Deigni OA, Baumann DP, Adamson KA, et al. Immediate Contralateral Mastopexy/Breast Reduction for Symmetry Can Be Performed Safely in Oncoplastic Breast-Conserving Surgery. Plast Reconstr Surg 2020;145:1134-42. [Crossref] [PubMed]
- Losken A, Pinell-White X, Hart AM, et al. The oncoplastic reduction approach to breast conservation therapy: benefits for margin control. Aesthet Surg J 2014;34:1185-91. [Crossref] [PubMed]
- Amabile MI, Mazouni C, Guimond C, et al. Factors Predictive of Re-excision After Oncoplastic Breast-conserving Surgery. Anticancer Res 2015;35:4229-34. [PubMed]
- Clough KB, Gouveia PF, Benyahi D, et al. Positive Margins After Oncoplastic Surgery for Breast Cancer. Ann Surg Oncol 2015;22:4247-53. [Crossref] [PubMed]
- Chatterjee A, Yao M, Sekigami Y, et al. Practical perspectives regarding patient selection and technical considerations in oncoplastic surgery. Current Breast Cancer Reports 2019;11:35-42. [Crossref]
- Niinikoski L, Leidenius MHK, Vaara P, et al. Resection margins and local recurrences in breast cancer: Comparison between conventional and oncoplastic breast conserving surgery. Eur J Surg Oncol 2019;45:976-82. [Crossref] [PubMed]
- Haloua MH, Krekel NM, Winters HA, et al. A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects. Ann Surg 2013;257:609-20. [Crossref] [PubMed]
- Rose M, Svensson H, Handler J, et al. Patient-reported outcome after oncoplastic breast surgery compared with conventional breast-conserving surgery in breast cancer. Breast Cancer Res Treat 2020;180:247-56. [Crossref] [PubMed]
- Veiga DF, Veiga-Filho J, Ribeiro LM, et al. Quality-of-life and self-esteem outcomes after oncoplastic breast-conserving surgery. Plast Reconstr Surg 2010;125:811-7. [Crossref] [PubMed]
- Bazzarelli A, Baker L, Petrcich W, et al. Patient Satisfaction Following Level II Oncoplastic Breast Surgery: A Comparison with Mastectomy Utililizing the Breast-Q Questionnaire will be published in Surgical Oncology. Surg Oncol 2020;35:556-9. [Crossref] [PubMed]
- Chand ND, Browne V, Paramanathan N, et al. Patient-Reported Outcomes Are Better after Oncoplastic Breast Conservation than after Mastectomy and Autologous Reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1419. [Crossref] [PubMed]
- Hart AM, Pinell-White X, Egro FM, et al. The Psychosexual Impact of Partial and Total Breast Reconstruction: A Prospective One-Year Longitudinal Study. Ann Plast Surg 2015;75:281-6. [Crossref] [PubMed]
- Char S, Bloom JA, Erlichman Z, et al. A comprehensive literature review of patient-reported outcome measures (PROMs) among common breast reconstruction options: What types of breast reconstruction score well? Breast J 2021;27:322-9. [Crossref] [PubMed]
- Gardfjell A, Dahlbäck C, Åhsberg K. Patient satisfaction after unilateral oncoplastic volume displacement surgery for breast cancer, evaluated with the BREAST-Q™. World J Surg Oncol 2019;17:96. [Crossref] [PubMed]
- Kim KD, Kim Z, Kuk JC, et al. Long-term results of oncoplastic breast surgery with latissimus dorsi flap reconstruction: a pilot study of the objective cosmetic results and patient reported outcome. Ann Surg Treat Res 2016;90:117-23. [Crossref] [PubMed]
- van Paridon MW, Kamali P, Paul MA, et al. Oncoplastic breast surgery: Achieving oncological and aesthetic outcomes. J Surg Oncol 2017;116:195-202. [Crossref] [PubMed]
- Losken A, Hart AM, Broecker JS, et al. Oncoplastic Breast Reduction Technique and Outcomes: An Evolution over 20 Years. Plast Reconstr Surg 2017;139:824e-33e. [Crossref] [PubMed]
- Ojala K, Meretoja TJ, Leidenius MH. Aesthetic and functional outcome after breast conserving surgery - Comparison between conventional and oncoplastic resection. Eur J Surg Oncol 2017;43:658-64. [Crossref] [PubMed]
Cite this article as: Thompson PW, Chatterjee A, Losken A. Standards in oncoplastic breast-conserving surgery. Ann Breast Surg 2022;6:37.

