
Case report of stacked intercostal artery perforator flaps: a novel technique using anterior and lateral intercostal artery perforator flaps for full autologous breast reconstruction post-mastectomy
IntroductionOther Section
Surgery is the mainstay of curative treatment for breast cancer with mastectomy offering the lowest risk of local recurrence (1,2). Post-mastectomy reconstruction options include autologous flaps and/or implants (3,4). Autologous flaps are commonly harvested from various areas, including rectus abdominis muscle, latissimus dorsi muscle, gracilis muscle and gluteal areas (5). While good cosmetic outcomes are often achieved, donor site morbidity and long patient downtime remain an issue (6). The intercostal perforator flaps have fewer of these problems but it often does not have sufficient volume for full reconstruction after mastectomy (7,8). Volume replacement techniques after breast-conserving surgery using perforator flaps commonly include the anterior intercostal artery perforator (AICAP) and lateral intercostal artery perforator (LICAP) flaps (9,10). LICAP flap originates from perforators arising from the costal segment while AICAP flap originates from perforators arising from the muscular segment (9). These perforator flaps have not been used for full reconstruction following a mastectomy in view of their smaller bulk.
We present our experience in using a combination of the AICAP flap with the LICAP flap (STICAP flaps) in a patient for full post-mastectomy autologous reconstruction. To the best of our knowledge, this is the first reported case of a STICAP flaps reconstruction following areolar-sparing mastectomy with excellent aesthetic outcome. We present the following article in accordance with the CARE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-79/rc).
Case presentationOther Section
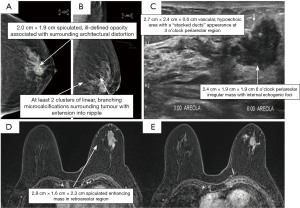
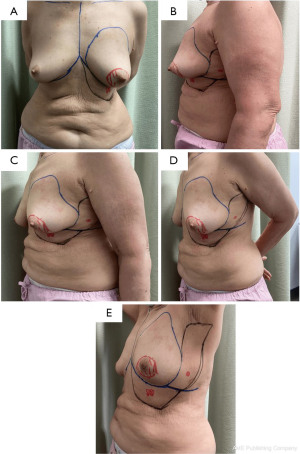
Our patient is a 76-year-old lady who presented with a palpable 2.0 cm × 1.9 cm firm mobile left breast lump at the 6 o’clock peri-areolar region. Imaging showed a large spiculated enhancing mass in the left retro-areolar region measuring 2.8 cm × 1.6 cm × 2.3 cm (Figure 1). There was clumped non-mass enhancement superior to the tumour in the central breast. Clumped linear non-mass enhancement was also noted in the central breast medial and lateral to the tumour and near the inferior aspect of the tumour. Hence, the collective size of the enhancing mass and surrounding, suspicious non-mass enhancement areas measured approximately 5.5 cm × 2.9 cm × 3.8 cm and occupied the whole retro-areolar and inferior half of the breast. In addition, nodular linear enhancement towards the left nipple was suspicious of ductal extension and nipple involvement. Ultrasound-guided core biopsy of the left breast lump confirmed invasive ductal carcinoma with negative estrogen receptor/progesterone receptor (ER/PR) status and positive human epidermal growth factor receptor 2 (HER2) status. Staging scans were negative for distant metastases. The patient was offered the options of upfront curative surgery versus neoadjuvant chemotherapy followed by curative surgery in view of tumour biology. All surgical options were offered and discussed in detail. The patient was not keen for a long and unsightly abdominal donor site scar and was assessed to have inadequate back tissue for a full reconstruction post-mastectomy. However, she had substantial tissue bulk at the AICAP and LICAP perforators region for full reconstruction by combining the two flaps. Pre-operative ultrasound doppler assessment demonstrated excellent doppler signals for both anterior and LICAP vessels. Pre-operative photos are shown in Figure 2.
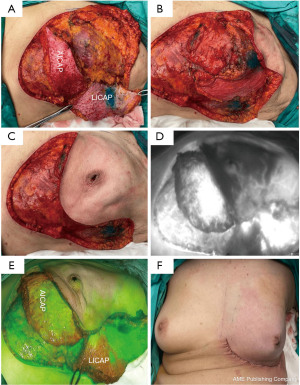
Our patient underwent a left breast areolar-sparing mastectomy and axillary sampling followed by immediate STICAP (AICAP and LICAP) flaps reconstruction. An infra-mammary fold (IMF) incision with lateral extension was used for the left areolar-sparing mastectomy and sentinel lymph node biopsy. For STICAP flaps harvest, dissection is carried down to the chest wall from the same IMF incision used for the mastectomy. Following identification of the latissimus, dissection continues above the muscle fascia from medial to lateral (LICAP) and cranial to caudal (AICAP). Subsequently, the fasciocutaneous flaps were dissected off the chest wall towards the anterior axillary line in the suprafascial plane. Thereafter, both AICAP and LICAP flaps were raised based on their respective perforators, identified pre-operatively with ultrasound doppler. Following which, indocyanine green (ICG) fluorescence imaging was used to assess the vascularity of the flaps using the pure fluorescence and overlay modes. ICG fluorescence imaging assessment demonstrated good vascularity of the STICAP flaps as well as the mastectomy skin flaps. The STICAP flaps were de-epithelised and rotated into the mastectomy cavity, fashioned and inset with absorbable stitches. Additional tacking sutures were placed to secure the flap to the anterior chest wall and avoid torsion of the perforator pedicle. The IMF was recreated with absorbable stitches (Figure 3). The surgery was uneventful, the patient recovered well and went home with two surgical drains. She was reviewed in the outpatient clinic at regular intervals until both drains were removed.
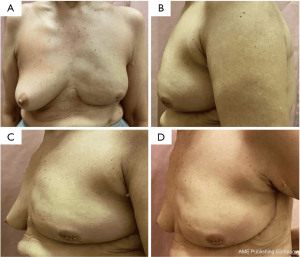
The wound healed well with a satisfactory aesthetic appearance as shown in Figure 4. The patient expressed a high level of satisfaction with the outcome. Histology revealed a 40 mm invasive ductal carcinoma with extensive high-grade ductal carcinoma in-situ, grade 3, five axillary lymph nodes were negative, stage 2A (pT2N0M0). A multidisciplinary discussion held recommended for her to receive adjuvant chemotherapy and targeted therapy.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
DiscussionOther Section
Full reconstruction with autologous tissue post-mastectomy is frequently the preferred option. However, there are several disadvantages with autologous reconstruction. These include but are not limited to a long and unsightly donor scar, donor site morbidity and longer recovery duration (11,12). In addition, free flap reconstruction has several associated morbidities (13). These include higher risk of vascular compromise of the flap, fat necrosis, wound infection and dehiscence. In addition, abdominal wall hematomas or seromas can occur which may occasionally require drainage or further surgical intervention (14).
In contrast, intercostal perforator flaps have less morbidity as it does not sacrifice the underlying muscle compared with myocutaneous flaps and can be considered as an attractive alternative to free flap reconstruction. These flaps were originally developed for partial reconstruction after breast-conserving surgery and not for full reconstruction due to their small volume. The original blood vessel supply to perforator flaps come off on the deep surface of the muscle supplying overlying skin as well as fat through musculocutaneous perforators (15). The flaps comprising skin and fat alone may be obtained from various anatomic areas by delicately dissecting these perforating vessels as they travel through the muscle without needing to sacrifice muscle (16). Furthermore, these techniques provide an ideal option for breast reconstruction in women with small to moderate size breasts. Moreover, this prevents the need to harvest the latissimus dorsi flap in these patients. As such, ICAP flaps present an excellent alternate option for breast reconstruction with minimal morbidity and positive aesthetic outcomes. Various perforator flaps used for partial breast reconstruction after breast-conserving surgery include thoracodorsal artery perforator (TDAP), lateral thoracic artery perforator (LTAP), AICAP and LICAP (17,18). The LICAP flap originates from perforators arising from the intercostal segment of the intercostal vessels which are usually located in fifth to seventh intercostal spaces between 2.5 and 3.5 cm medial to the anterior border of the latissimus dorsi muscle (19). The LICAP flap is the most appropriate option for lateral quadrant defects. In addition, the scar following LICAP flap reconstruction is hidden under the arm and less visible. The LICAP flap could be an alternative to the TDAP flap as it provides ease of identification of perforators through anterior dissection (20). In contrast, the AICAP flap originates from perforators arising from the intercostal vessels through external oblique or rectus abdominus muscles typically located within 1 to 3 cm lateral to the sternal border (21). For the correction of small defects, the AICAP flaps have been described in a V-Y or propeller manner. It is well-suited to cover defects that extend over the medial or inferior quadrants for which other volume displacement or replacement techniques are challenging as it has a short pedicle (22).
To the best of our knowledge, this is the first reported case on the use of STICAP flaps for full autologous reconstruction post-mastectomy. It is novel to combine two intercostal flaps together to obtain sufficient volume for full reconstruction post-mastectomy.
The advantage of the STICAP flaps is that both perforator flaps were raised through the same scar, hence minimising donor site morbidity and post-operative pain. By combining two perforator flaps, the STICAP flaps allowed for sufficient volume to achieve full post-mastectomy reconstruction. Together with the well-hidden scar, good cosmetic outcome is achieved.
However, as with all autologous flaps, the STICAP flaps carry an associated risk of fat necrosis due to possible venous congestion (23). A case series by Kim et al. included 40 patients that underwent LICAP flap by either the propeller method or the turnover method. Notably, venous congestion occurred in two cases of the propeller method and three cases needed treatment for fat necrosis (24). ICG dye-based fluorescent angiography is a useful technique for the evaluation of vascularity and tissue perfusion in skin flaps. Intra-operative ICG fluorescence imaging has been used for perfusion mapping of flaps, to predict and prevent flap complications (25,26). In our centre, flap perfusion is routinely assessed using ICG intra-operatively. In the event of poor perfusion noted intra-operatively, flap tissue shown to be poorly perfused will be trimmed and fashioned prior to final inset to reduce risk of subsequent fat necrosis and its associated complications.
Due to the relatively short follow-up in our case, long- term aesthetic outcomes need to be ascertained. Although this technique provides a good breast mound, symmetry with the contralateral breast may not be the most ideal. Mobilisation of the perforator flaps creates an asymmetry in the upper abdominal area and lateral aspect of patient’s chest which may reduce patient satisfaction in certain patients. Moving forward, further validation of this technique and its long-term outcomes could be evaluated in a larger group of patients in a case series. In addition, lipofilling may be offered to address any asymmetry or volume deficit in patients who might require it. This will be assessed after at least 6 months after completion of treatment to allow for complete tissue remodeling to take place.
ConclusionsOther Section
In conclusion, our experience with the STICAP (combined AICAP and LICAP) flaps for full post-mastectomy reconstruction demonstrates that it is a feasible option which can provide favourable oncological and cosmetic outcomes. Careful patient selection, detailed pre-operative planning and flap design are essential to achieving an ideal outcome.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-79/rc
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-79/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-79/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Zumsteg ZS, Morrow M, Arnold B, et al. Breast-conserving therapy achieves locoregional outcomes comparable to mastectomy in women with T1-2N0 triple-negative breast cancer. Ann Surg Oncol 2013;20:3469-76. [Crossref] [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Chao AH, Garza R 3rd, Povoski SP. A review of the use of silicone implants in breast surgery. Expert Rev Med Devices 2016;13:143-56. [Crossref] [PubMed]
- Hamdi M, Van Landuyt K, Monstrey S, et al. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg 2004;57:531-9. [Crossref] [PubMed]
- Yang JD, Lee JW, Kim WW, et al. Oncoplastic surgical techniques for personalized breast conserving surgery in breast cancer patient with small to moderate sized breast. J Breast Cancer 2011;14:253-61. [Crossref] [PubMed]
- Hamdi M, Rasheed MZ. Advances in autologous breast reconstruction with pedicled perforator flaps. Clin Plast Surg 2012;39:477-90. [Crossref] [PubMed]
- Yang JD, Kim MC, Lee JW, et al. Usefulness of Oncoplastic Volume Replacement Techniques after Breast Conserving Surgery in Small to Moderate-sized Breasts. Arch Plast Surg 2012;39:489-96. [Crossref] [PubMed]
- Hamdi M, De Frene B. Pedicled perforator flaps in breast reconstruction. Semin Plast Surg 2006;20:73-8. [Crossref]
- Hamdi M, Van Landuyt K, de Frene B, et al. The versatility of the inter-costal artery perforator (ICAP) flaps. J Plast Reconstr Aesthet Surg 2006;59:644-52. [Crossref] [PubMed]
- Santanelli F, Longo B, Germano S, et al. Total breast reconstruction using the thoracodorsal artery perforator flap without implant. Plast Reconstr Surg 2014;133:251-4. [Crossref] [PubMed]
- Macadam SA, Bovill ES, Buchel EW, et al. Evidence-Based Medicine: Autologous Breast Reconstruction. Plast Reconstr Surg 2017;139:204e-29e. [Crossref] [PubMed]
- Glynn C, Litherland J. Imaging breast augmentation and reconstruction. Br J Radiol 2008;81:587-95. [Crossref] [PubMed]
- Chevray PM. Update on Breast Reconstruction Using Free TRAM, DIEP, and SIEA Flaps. Semin Plast Surg 2004;18:97-104. [Crossref] [PubMed]
- Andrades P, Fix RJ, Danilla S, et al. Ischemic complications in pedicle, free, and muscle sparing transverse rectus abdominis myocutaneous flaps for breast reconstruction. Ann Plast Surg 2008;60:562-7. [Crossref] [PubMed]
- Heitmann C, Guerra A, Metzinger SW, et al. The thoracodorsal artery perforator flap: anatomic basis and clinical application. Ann Plast Surg 2003;51:23-9. [Crossref] [PubMed]
- Guerra AB, Metzinger SE, Lund KM, et al. The thoracodorsal artery perforator flap: clinical experience and anatomic study with emphasis on harvest techniques. Plast Reconstr Surg 2004;114:32-41; discussion 42-3. [Crossref] [PubMed]
- Van Landuyt K, Hamdi M, Blondeel P, et al. Autologous breast augmentation by pedicled perforator flaps. Ann Plast Surg 2004;53:322-7. [Crossref] [PubMed]
- Levine JL, Soueid NE, Allen RJ. Algorithm for autologous breast reconstruction for partial mastectomy defects. Plast Reconstr Surg 2005;116:762-7. [Crossref] [PubMed]
- Munhoz AM, Montag E, Arruda E, et al. Immediate conservative breast surgery reconstruction with perforator flaps: new challenges in the era of partial mastectomy reconstruction? Breast 2011;20:233-40. [Crossref] [PubMed]
- Griffiths M, Chae MP, Rozen WM. Indocyanine green-based fluorescent angiography in breast reconstruction. Gland Surg 2016;5:133-49. [PubMed]
- Hakakian CS, Lockhart RA, Kulber DA, et al. Lateral Intercostal Artery Perforator Flap in Breast Reconstruction: A Simplified Pedicle Permits an Expanded Role. Ann Plast Surg 2016;76:S184-90. [Crossref] [PubMed]
- Kerrigan CL, Daniel RK. The intercostal flap: an anatomical and hemodynamic approach. Ann Plast Surg 1979;2:411-21. [Crossref] [PubMed]
- Katira K, Goyal S, Venditto C, et al. Successful Salvage of Delayed Venous Congestion After DIEP Flap Breast Reconstruction. Eplasty 2019;19:e22. [PubMed]
- Kim JB, Kim DK, Lee JW, et al. The usefulness of pedicled perforator flap in partial breast reconstruction after breast conserving surgery in Korean women. Arch Plast Surg 2018;45:29-36. [Crossref] [PubMed]
- Matsui A, Lee BT, Winer JH, et al. Quantitative assessment of perfusion and vascular compromise in perforator flaps using a near-infrared fluorescence-guided imaging system. Plast Reconstr Surg 2009;124:451-60. [Crossref] [PubMed]
- Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg 2010;125:1065-73. [Crossref] [PubMed]
Cite this article as: Lim HJ, Mok CW, Tan SM. Case report of stacked intercostal artery perforator flaps: a novel technique using anterior and lateral intercostal artery perforator flaps for full autologous breast reconstruction post-mastectomy. Ann Breast Surg 2023;7:9.

