
Resource utilization and patient reported outcomes using acellular dermal matrix in breast reconstructive procedures
Introduction
In the western world an increasing part of breast reconstructive procedures is being performed in an increasingly younger population (1,2). This leads to an awareness of improving the psychosocial and functional result and reduce the resource utilization as more women will live for a longer time with the consequences of breast cancer treatment.
In 2005/2006 Breuing and Salzberg were the first to publish the use of acellular dermal matrix (ADM) for immediate implant-based breast reconstruction (BR) following skin-sparing mastectomy (3,4) and in 2007 Bindingnavele et al. introduced the use of ADM in tissue expander BR proposing that this would decrease the postoperative pain and allow a faster expansion course (5).
Limited health resources necessitate careful consideration of the implementation of a given treatment modality. ADM products are expensive but may potentially be cost-effective, due to the possibility of reducing expenses as i.e., fewer surgeries and shorter hospital stay, compared to the traditional two-stage expander-implant technique. The literature regarding this subject shows conflicting results. Some suggest that the use of ADM for immediate BR is cost advantageous compared with the two-stage approach and furthermore, that the use of ADM has clinical benefit for patients by allowing a one-stage procedure rather than two separate operations and results in fewer outpatient visits (6,7). Another study has reported that the direct costs of one-stage implant-based BR with ADM were higher than those of two-stage BR, and that health outcomes did not differ between the groups (8).
The advantages of using ADM in BR are improved control of the inframammary fold position (9) and better lower pole projection (10) compared to the traditional expander-to-implant technique. Furthermore, studies indicate that implant-based BR with ADM results in a lower rate of development of capsular contracture, even when the patient has to undergo radiation therapy (11,12). Seroma has, on the other hand, been associated with the use of biological meshes (13,14). The patient’s subjective assessment of the aesthetic outcome and the physical and psychosocial effects of BR is extremely important as the overall objective by offering BR is to improve the patients quality of life (QoL).
To contribute to knowledge on the subject, the present study aims at comparing immediate implant-based BR using the one-stage approach with ADM with the two-stage expander-to-implant approach regarding resource utilization and patient reported outcomes (PROs). We present the following article in accordance with the STROBE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-81/rc).
Methods
Study design and participants
The present study was designed as an observational cohort study with 44 participants. Eligible patients were all women admitted for immediate, implant-based BR following skin-sparing mastectomy at the Department of Plastic and Breast Surgery, Aarhus University Hospital, Denmark over a period of 40 months. Patients were diagnosed with either breast cancer, ductal carcinoma in situ (DCIS) or were considered high risk for developing breast cancer. Inclusion criteria were mastectomy weight ≤600 g, patient older than 18 years, tobacco abstinence >4 weeks prior to surgery, ability to complete the study questionnaire, and for the two-stage group; time to achieve 2-year follow-up visit after BR. Follow-up visits were planned 12 and 24 months after insertion of silicone implant where patients completed a study-specific questionnaire regarding PROs. Furthermore, a systematic review of patient records was performed to obtain information for analysis regarding resource utilization. Follow-up time was 24 months.
All participants gave written informed consent. The Ethics Committee of the Central Region of Denmark (1-10-72-572-12) approved this study and it was submitted in ClinicalTrials.gov (NCT04209010). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Recruitment
As the one-stage approach was implemented as a standard care for immediate implant-based BR following skin-sparing mastectomy, in December 2012, all eligible patients were offered participation in the one-stage group and inclusion continued consecutively until 21 patients were included. The two-stage cohort was established retrospectively. Patients that had undergone immediate implant-based BR following skin-sparing mastectomy with the two-stage expander-to-implant technique were identified using diagnosis- and procedure-related codes, records were examined and patients that fulfilled the inclusion criteria were identified and consecutively offered participation in the two-stage group. Inclusion continued retrospectively until 23 patients were included (see Figure S1). The same study population has been used for the publication entitled “Comparison of one-stage direct-to-implant with acellular dermal matrix and two-stage immediate implant-based breast reconstruction-a cohort study” (15) where the outcome was postoperative complications, aesthetic correction procedures and aesthetic outcome.
Study size
Study size was determined upon power calculation on the primary endpoint “reduction in surgery time” as the duration of surgery was considered to have a significant impact on overall resource utilization. Duration of surgery time for bilateral BR with the two-stage technique was, based on own experience, estimated to 300 minutes. The minimum relevant difference the study was aiming to achieve was 60 minutes reduction in surgery time using the one-stage technique (16). With a significance level at 5% and power on 80%, it was calculated that 16 patients were needed in each treatment group. Originally 20 patients were planned in each group, but late secondary review of patients revealed, that one patient had been excluded by mistake from the one-stage group due to conversion to expander-based BR because of vulnerable mastectomy flaps and another three patients were excluded due to removal of implant before inclusion started in the two-stage group. Allocating these patients to their correct study group resulted in 21 patients in the one-stage group and 23 patients in the two-stage group.
Surgical techniques
The surgical technique for one-stage ADM assisted immediate BR and for expander to implant two-stage immediate BR was described in a previous published paper (15). No patients underwent postoperative radiation therapy.
Outcomes
The primary endpoint of the study was resource utilization reported for bilateral and unilateral BRs in the two treatment arms. It was not possible to assign a monetary value on all variables, but the assumption was made that if e.g., number of interventions were higher in one group compared to the other, this would lead to increased resource utilization. The following variables were included: (I) cost of silicone implants, sizers, expanders and sheets of ADM (StratticeTM pliable 8×16 cm) in €. (II) Duration of the breast reconstructive procedure in minutes. In case of unilateral BR with contralateral breast surgery in the same intervention, the duration of the breast reconstructive procedure was estimated by a senior consultant (TD). (III) Number of outpatient visits for expansions in patients who underwent a two-stage procedure. (IV) Number of interventions to address seroma. (V) Number of surgical interventions to address complications. In case several procedures were done during the same surgery it only counted for one intervention. (VI) Number of surgical interventions to address aesthetic outcome. In case of unilateral BR with contralateral breast surgery at the same time as the breast reconstructive procedure, the contralateral procedure counted for one aesthetic intervention. (VII) Duration of hospitalization in days and estimated costs in € and (VIII) duration of sick leave reported by patients (counted as days before work was resumed). These data were obtained for a 2-year period after insertion of the final-size silicone implant. All second stage surgeries for the two-stage group were completed.
Secondary endpoints were PRO measures (PROMs) including Hopwoods body image scale (BIS) and a study specific questionnaire.
Body image was evaluated using Hopwoods BIS (17) at 12- and 24-month follow-up. The scale is validated for use in breast cancer patients and consists of 10 items answered with reference to the past week. The scale has high reliability, good clinical validity, and is sensitive to changes. Items include evaluation of femininity, self-consciousness, physical and sexual attractiveness, and satisfaction with body and scars. Each question has four options for rating body image: “not at all” (score 0), “a little” (score 1), “quite a bit” (score 2) and “very much” (score 3). The 10 item scores were summed to produce an overall score for each patient, ranging from 0 to 30, with 0 representing no symptom/distress and higher scores representing increasing symptoms/distress.
Furthermore, the patients fulfilled a study specific questionnaire regarding health status, QoL, pain, sensory disturbance and functional sequalae at 12- and 24-month follow-up consisting of items answered at breast level and at patient level. Some of the questions were answered on a scale and were dichotomized prior to analysis as elaborated in the description of questions found in Appendix 1.
Bias
The funders (financial or the ADM supplier) did not participate in study design, data collection, data analysis, or interpretation and writing of the manuscript.
Statistical analysis
Descriptive statistics were used for patients’ demographics with mean and standard deviation for continuous variables. Categorical variables were compared between study arms using Fisher’s exact test while continuous variables were compared by a t-test.
For the resource utilization analysis part, simple linear regression models were used and uni- and bilateral BR were compared separately between the treatment groups. BIS was analyzed using a mixed regression model due to repeated measurements using patient ID as random effect. Due to the small sample size, the Kenward Roger approximation method was used to calculate the degrees of freedom. The regression model assumptions were checked by visual inspection of the diagnostics plots such as QQ plot for the residuals and the scatter plot of residuals and the fitted values. If necessary, a log-transformed outcome was modelled.
PROMs reported at patient level with dichotomized outcomes were analyzed using generalized linear models with log-link function adjusting for repeated measurements by using patient ID as cluster. Regarding health-related limitation of activities the sum score was analyzed using a mixed model, adjusting for the repeated measurements and small sample size as described above.
The original outcomes of PROMs reported at breast level had flooring effect (except the question: Do you feel burdened by sensory disturbances in the area where you were operated?) i.e., many of the answers were “no pain” or similar to that. Therefore, all the outcomes were dichotomized as “no pain” or “yes, pain” (or similar). PROMs reported at breast level with binary outcome were analyzed using a generalized linear model with log-link function. By keeping the smaller sample size in mind, especially those with bilateral surgery, the two breasts were assumed to be coming from two different patients. Therefore, a new ID variable was created at the breast level, assuming that every BR is from one individual, and used as clusters in the model to adjust for the repeated measurements.
The patient (in case of unilateral BR or bilateral BR with bilateral explantation) or the breast (in case of bilateral BR with unilateral explantation) was categorized as lost to follow-up if explantation occurred. Therefore, some patients did not have the opportunity to answer the questionnaire at follow-up visits and were thereby not randomly missing. This was the case for five patients (nine breasts) in the one-stage group and four patients (seven breasts) in the two-stage group (Figure 1).
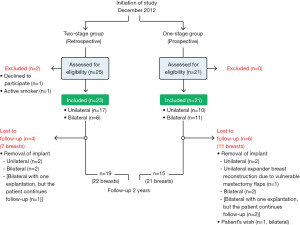
Statistical analyses were performed using STATA® software IC16.1 (Stata Corporation, College Station, TX, USA). Strobe guidelines for reporting observational cohort study were used.
Results
Forty-four patients were included in the study, 21 patients (32 breasts) in the one-stage group and 23 patients (29 breasts) in the two-stage group. Fifteen patients (21 breasts) in the one-stage group and 19 patients (22 breasts) in the two-stage group completed 24-month follow-up (Figure 1). The two groups did not differ significantly regarding demographics and clinical characteristics as summarized in Table 1.
Table 1
| Variables | One-stage, n=21 | Two-stage, n=23 |
|---|---|---|
| Age (years), mean (SD) | 48.3 (10.7) | 42.7 (9.9) |
| BMI (kg/m2), mean (SD)† | 23.1 (2.8) | 24.7 (3.8) |
| Comorbidity†, n | 7 | 3 |
| Laterality of procedure, n | ||
| Bilateral | 11 | 6 |
| Unilateral | 10 | 17 |
| Adjuvant therapy after surgery†, n | ||
| Endocrine treatment | 5 | 1 |
| None | 15 | 19 |
| Axillary surgery†, n | ||
| None | 13 | 13 |
| Sentinel node biopsy | 7 | 5 |
| Axillary dissection‡ | 0 | 2 |
| Indication for mastectomy†, n | ||
| Cancer | 3 | 0 |
| DCIS | 2 | 6 |
| Prophylactic | 15 | 14 |
†, missing values one-stage group n=1, two-stage group n=3; ‡, two patients in the two-stage group were diagnosed with DCIS but underwent axillary dissection due to micrometastasis in sentinel nodes. BMI, body mass index; DCIS, ductal carcinoma in situ.
Regarding the primary endpoint “resource utilization” associated with the two different methods for BR the materials for a one-stage BR (silicone implant, StratticeTM, sizer) was 2.6 times more expensive than materials for a two-stage BR (expander, silicone implant, sizer ×2) with a 1,795 € difference in costs for a unilateral procedure (Table 2).
Table 2
| Variables | Unilateral | Bilateral | |||||||
|---|---|---|---|---|---|---|---|---|---|
| One-stage, n=10 | Two-stage, n=17 | Comparison | P | One-stage, n=11 | Two-stage, n=6 | Comparison | P | ||
| Total cost of materials (€)† | 2.935 | 1.140 | 5.870 | 2.280 | |||||
| Duration of operation (min)‡ | |||||||||
| First operation | 136 (116–160) | 95 (80–113) | 225 (200–253) | 151 (112–205) | |||||
| Second operation | 83 (67–103) | 93 (64–135) | |||||||
| Overall | 136 (116–160), M=1 | 183 (162–207), M=2 | 1.34 (1.10–1.65) | 0.006* | 225 (200–253) | 247 (207–295), M=1 | 1.10 (0.89–1.36) | NS | |
| Expansions§ | 6.3 (5.2–7.3), (range, 3–11), M=2 | 5.9 (4.1–7.7), (range, 4–8.5), M=1 | |||||||
| Interventions to address seroma§ | 0.11 (−0.28 to 0.51), M=1 | 0.24 (−0.05 to 0.52) | 0.12 (−0.37 to 0.61) | NS | 0 (−0.15 to 0.15) | 0.17 (−0.04 to 0.37) | 0.17 (−0.09 to 0.42) | NS | |
| Surgical interventions to address complications§ | 0.56 (−0.002 to 1.11), M=1 | 0.29 (−0.11 to 0.7) | −0.26 (−0.95 to 0.43) | NS | 1 (0.20–1.8) | 0.5 (−0.59 to 1.59) | −0.5 (−1.85 to 0.85) | NS | |
| Surgical interventions to address aesthetic outcome§ | 1.57 (1.05–2.09), M=3 | 0.27 (−0.09 to 0.62), M=2 | −1.3 (−1.93 to 0.68) | <0.0001* | 0.88 (0.28–1.47), M=3 | 0.75 (−0.09 to 1.59), M=2 | −0.13 (−1.15 to 0.9) | NS | |
| Duration of hospital stay (days)§ | |||||||||
| First operation | 10.4 (9.2–11.7) | 6.9 (6.2–7.7) | 12.1 (10.4–13.8) | 7 (5.7–8.3) | |||||
| Second operation | 3.2 (2.7–3.7) | 2.6 (1.7–3.5) | |||||||
| Overall | 10.4 (9.2–11.7), M=1 | 10.1 (9.2–11.1), M=2 | −0.3 (−1.9 to 1.3) | NS | 12.1 (10.4–13.8) | 9.6 (7–12.2), M=1 | −2.5 (−5.6 to 0.6) | NS | |
| Total cost for hospitalization, 470 € per day§ | 4,909 (4,330–5,488), M=1 | 4,763 (4,314–5,211), M=2 | −146 (−879 to 587) | NS | 5,683 (4,870–6,495) | 4,512 (3,306–5,718), M=1 | −1,171 (−2,625 to 283) | NS | |
| Sick leave (days)§ | 40.5 (9.2–71.8), M=6 | 42.3 (23.4–61.2), M=6 | 1.8 (−34.8 to 38.3) | NS | 62.6 (39.8–85.4), M=3 | 59.5 (13.9–105.1), M=4 | −3.1 (−54.1 to 47.8) | NS | |
*, statistically significant P value; †, one-stage group (silicone implant, StratticeTM, sizer), two-stage group (expander, silicone implant, sizer ×2); ‡, median (95% CI). Comparison with the ratio of medians (95% CI, P) with reference to the one-stage group; §, mean (95% CI). Comparison with the difference (95% CI, P) with reference to the one-stage group. BR, breast reconstruction; M, number of missing data; NS, not significant.
The one-stage procedure took longer time than the first operation for the two-stage procedure for both unilateral and bilateral cases. But when the duration of procedures in the two-stage group were summed, the overall surgery time of a unilateral two-stage procedure was 34% longer than a one-stage procedure (P=0.006). For the bilateral groups the overall two-stage procedure took 10% longer (P=0.348) time than the one-stage procedure.
Patients undergoing BR with the two-stage method underwent in average 6.3 (unilateral) and 5.9 (bilateral) expansions. There was no statistically significant difference in mean number of interventions to address seroma between the two treatment groups.
For the variable “surgical interventions to address complications” a flooring effect was observed. For the unilateral one-stage group 7 of 9 patients (78%) and 13 of 17 patients (76%) in the two-stage group did not undergo any surgeries due to complications. For the bilateral groups, 6 of 11 patients (55%) and 4 of 6 patients (67%) did not undergo any surgeries due to complications, respectively. By calculating mean number of interventions to address complications there were no significant difference between the two treatment groups for either unilateral BR nor bilateral BR.
Twelve of 15 patients (80%) (2 missing) in the unilateral two-stage group did not undergo further surgical procedures to address aesthetic outcome. With comparison to the one-stage group, where all 7 patients (100%) (3 missing) underwent at least one procedure to address aesthetic outcome, there was a statistically significant difference when comparing the means (P<0.0001). In the bilateral groups there was no significant difference between the mean number of interventions to address aesthetic outcome (P=0.791). By assuming that an intervention entails an expense the significant difference between the unilateral groups leads to the assumption that there are more expenses in the one-stage group.
Duration of overall hospital stay was the same for the two unilateral treatment groups (10 days) but 2 days longer for the bilateral one-stage patients (12 days) compared to the two-stage patients (10 days). There was no significant difference in self-reported sick leave between treatment arms.
Results concerning the secondary endpoint PRO are described as follows. Attention is drawn to the proportion of missing data especially at 12 months follow-up in the two-stage group and results are provided for 24-month follow-up (Table 3). Regarding pain located to the breast region there was no significant difference between groups at 24-month follow-up (RR: 1.67, P=0.354). Nor was there any significant difference within the groups between 12- and 24-month follow-up. Patients were in general mildly burdened by sensory disturbances in the operation field as the means for the outcome (where the outcome is scaled from 1= minimum burden to 5= extreme burden) at 24 months were 1.52 (SD: 1.12) and 1.33 (SD: 1.09) for one- and two-stage group, respectively. There was no statistically significant difference between groups at 24-month follow-up (RR: 0.90, 95% CI: 0.68–1.20, P=0.482) or within groups between 12- and 24- months follow-up. Considering patient reported pain in the arm or shoulder on the operated side no statistically significant differences were observed within the groups between 12- and 24-month follow-up in either of the treatment groups. Even though more pain in the arm or shoulder was reported at 24-month follow-up in the two-stage group (32%) this was not statistically significant different from the one-stage group (14%, P=0.201). Regarding sensory disturbances in the arm or shoulder on the operated side there was no statistically significant difference reported within the groups between 12- and 24-month follow-up in either of the treatment groups or between groups at 24-month follow-up (RR: 1.33, P=0.566).
Table 3
| Variables | One-stage, n=32† | Two-stage, n=29† | RR | P | |||
|---|---|---|---|---|---|---|---|
| 12 months | 24 months | 12 months | 24 months | ||||
| Have you felt pain in the area where you were operated? | |||||||
| Yes¶ | 6 (29%; 14–57%) | 4 (19%; 8–47%) | 2 (25%; 7–84%) | 7 (32%; 17–59%) | 1.67 (0.56–4.94) | NS | |
| Do you feel burdened by sensory disturbances in the area where you were operated? | |||||||
| Yes¶ | 18 (86%; 72–102%) | 18 (86%; 72–102%) | 4 (50%; 25–101%) | 17 (77%; 61–97%) | 0.90 (0.68–1.20) | NS | |
| Mean (SD) | 1.38 (0.97) | 1.52 (1.12) | 1.50 (1.85) | 1.33 (1.09) | |||
| Have you felt pain in the arm or shoulder on the operated side? | |||||||
| Yes¶ | 3 (15%; 5–43%), M=12 | 3 (14%; 5–41%) | 2 (25%; 7–84%) | 7 (32%; 17–59%) | 2.2 (0.65–7.60) | NS | |
| Do you feel burdened by sensory disturbances in the arm or shoulder on the operated side? | |||||||
| Yes¶ | 3 (15%; 5–43%), M=12 | 5 (24%; 11–52%) | 4 (57%; 30–109%) | 7 (32%; 17–59%) | 1.33 (0.50–3.60) | NS | |
| Do you suffer from lymphedema in the arm or hand on the operated side? | |||||||
| Yes, n | 1 | 0 | 0 | 2 | |||
| Are you able to use the arm on the operated side as before surgery? | |||||||
| Yes¶ | 15 (71%; 54–94%) | 21 | 7, M=22 | 18 (82%; 67–100%) | |||
†, missing values one-stage group: n=11 at 12- and 24-month follow-up. Two-stage group: n=21 at 12-month follow-up and n=7 at 24-month follow-up. Exception from this is M; ¶, n (proportion in %; 95% CI) and risk ratio (95% CI, P) for comparison of groups at 24-month follow-up with the one-stage group as reference. PROMs, patient reported outcome measures; M, number of missing data; NS, not significant; N, number.
One patient in the one-stage group reported lymphedema at 12-month follow-up and two patients in the two-stage group at 24-month follow-up. Both patients in the two-stage group underwent axillary dissection before unilateral BR.
All in the one-stage group (of 21 reported) and 82% (of 22 reported) in the two-stage group was able to use the arm on the operated side as before surgery at 24-month follow-up.
Body image improved (BIS score reduction) in the one-stage group from 12 months (6.9) to 24 months follow-up (5.6), although this was not significant (difference: −1.3 points; 95% CI: −3.2 to 0.5, P=0.144) (Table 4). In the two-stage group the mean BIS score was 5.6 at both 12- and 24-month follow-up with no significant difference (P=0.9888). Thereby, the reduction in mean BIS score from 12- to 24-month follow-up were not statistically significant between the groups (P=0.446).
Table 4
| Variables | One-stage, n=21† | Two-stage, n=23† | Comparison w.r.t. one-stage group | P | |||
|---|---|---|---|---|---|---|---|
| 12 months | 24 months | 12 months | 24 months | ||||
| BIS§,‡ | 6.9 (4.1–9.7) | 5.6 (2.8–8.4) | 5.6 (2.3–8.9) | 5.6 (3.1–8) | −0.01 (3.88–3.85) | NS | |
| Health related limitation of activities+,§ | 8.8 (7.9–9.7) | 8.9 (8–9.8) | 8.8 (7.8–9.9) | 8.4 (7.5–9.1) | −0.5 (−1.75 to 0.66) | NS | |
| How is your current overall health status? | |||||||
| Good¶ | 15 | 15 | 5 (83%; 58–120%) | 18 (95%; 85–106%) | |||
| How is your current overall health status compared to the time of BR? | |||||||
| Improved¶ | 5 (33%; 16–69%) | 7 (47%; 27–81%) | 1 (17%; 3–102%) | 7 (37%; 20–67%) | 0.8 (0.4–1.8) | NS | |
| Was BR the right choice for you? | |||||||
| Yes, n | 14 | 15 | 6 | 19 | |||
| With your current experience, would you recommend others to undergo BR? | |||||||
| Yes, n | 15 | 15 | 5 | 19 | |||
| How would you describe your current QoL compared to the time before your BR? | |||||||
| Improved¶ | 9 (60%; 40–91%) | 11 (73%; 54–100%) | 2 (33%; 11–105%) | 10 (53%; 34–81%) | 0.7 (0.4–1.2) | NS | |
| Have you been taking painkillers within the past month? | |||||||
| Yes¶ | 2 (13%; 4–49%) | 1 (7%; 1–46%) | 2 (33%; 11–105%) | 7 (37%; 20–67%) | 5.5 (0.7–41.3) | NS | |
| Pain elsewhere in the body? | |||||||
| Yes¶ | 4 (27%; 11–63%) | 3 (20%; 7–56%) | 0 | 5 (28%; 13–59%), M=5 | 1.39 (0.39–4.98) | NS | |
†, missing values one-stage group: n=6 at 12- and 24-month follow-up. Two-stage group: n=17 at 12-month follow-up and n=4 at 24-month follow-up. Exception from this is M; ‡, BIS range 0–30. 0 representing no symptom/distress and higher scores representing increasing symptoms/distress; §, mean (95% CI) and mean difference (95% CI, P) for comparison of groups at 24-month follow-up with reference to the one-stage group; ¶, n (proportion in %; 95% CI) and risk ratio (95% CI, P) for comparison of groups at 24-month follow-up with reference to the one-stage group; +, health related limitation of activities, range 0–10, higher scores representing more activities the patient can perform without any health-related limitations. PROMs, patient reported outcome measures; w.r.t., with reference to; BIS, body image scale; BR, breast reconstruction; QoL, quality of life; M, number of missing data; NS, not significant; N, number.
All patients were to a large degree unlimited in their ability to perform physical activities. In both treatment groups a mean score >8 (range, 0–10) at 12- and 24-month follow-up. There was no statistically significant difference between groups at 24-month follow-up (P=0.366).
Furthermore, all patients in the one-stage group and the far majority of patients in the two-stage group (83% and 95%) reported a good current overall health at 12- and 24-month follow-up. An increasing proportion of patients in both treatment groups report a better overall health status compared to the time of BR related to increasing time after surgery. The proportion of patients who report improved health was 27% (95% CI: 0.56–2.85) larger in the one-stage group compared to the two-stage group at 24-month follow-up (P=0.568).
All patients, except for one patient in the one-stage group at 12-month follow-up, thought that BR was the right choice for them and at 24-month follow-up all patients would recommend others in the same situation to undergo BR.
An increasing number of patients in both treatment groups experienced an improved QoL from 12 to 24 months postoperatively, though not statistically significant. Fifty-three percent of the patients in the two-stage group reported improved QoL at 24-month follow-up compared to 73% in the one-stage group (RR: 0.7, P=0.222).
The use of analgetics was reduced from 13% at 12-month follow-up to 7% at 24-month follow-up in the one-stage group but increased from 33% to 37% in the two-stage group. These changes within the groups were not statistically significant. More patients in the two-stage group had used painkillers within the past month compared to patients in the one-stage group at 24-month follow-up, though not statistically significant (RR: 5.5, P=0.096). At 24-month follow-up 20% in the one-stage group and 28% in the two-stage group report pain in other parts of the body than the operated area within the past month (P=0.614).
Discussion
The objective of this study was to compare two methods for immediate implant-based BR in a resource utilization analysis and furthermore, to discuss the result in conjunction with the patient’s subjective report of psychosocial and physical outcome measures.
Sample size of this study was determined upon an expected decrease in duration of surgery on 60 minutes when using the one-stage approach. However, the reduction was 47 minutes in the unilateral group and 22 minutes in the bilateral group and the assumptions made before study start was thereby not met. This leads to concerns whether it is possible to identify any differences between study groups because of sample size limitations. The conclusions to be drawn from the present study may also be limited by the retrospective inclusion of the two-stage group as no baseline measurements of PROs were obtained. Furthermore, the majority of patients in the two-stage group did not complete 12-month follow-up visit but only 24-month follow-up visit leading to a large proportion of missing data. Several additional variables would have been preferred in the resource utilization analysis. For example, total number of outpatient visits for both treatment groups, duration of surgery for additional surgeries due to complications and aesthetic outcome, prize setting of operation time etc. Furthermore, this study did not take into consideration the additional cost for another BR in the case of complications leading to implant loss. At the time of study start no validated Danish questionnaire, as BREAST-Q, for use in patients undergoing breast reconstructive procedures was available. Therefore, a study specific questionnaire was used including questions previously used at our institution (18). With these limitations in mind, the following overall thoughts about the outcome was proposed.
Healthcare cost can be calculated from different viewpoints including using reimbursement tariffs based on diagnosis related groups (DRGs) using average costing. This may not reflect the actual costing as shown by others (6,19) and in this publication the original variables as surgery time, number of additional surgeries and cost of materials were used.
Duration of surgery for the breast reconstructive procedure was longer in the two-stage group compared to the one-stage group (significant in the unilateral comparison) as found by others (8). During surgery for tissue expander-to-implant exchange adjustments such as implant pocket adjustments or revision of the inframammary fold, were often made and this could account for at least some of the extra time spent on surgery used in the two-stage group. This corresponds to the observation of more interventions for aesthetic corrections in the one-stage group compared to the two-stage group (significant in the unilateral comparison). If a one-stage BR ultimately requires additional interventions to obtain an aesthetically satisfying result, the advantage of completing the BR in a single stage is lost seen from both the patient and the hospital’s perspective. This paradox has also been noted by others (19,20). A major advantage of the one-stage approach is the possibility to avoid outpatient visits for expansion and the additional cost for outpatient clinic time and utensils may offset part of the cost of using ADM from the hospital’s perspective. For the patient there is a huge advantage in avoiding expansions as there are also many indirect costs as sick leave from job, discomfort, risk for adverse events, and the psychological burden of not having completed the BR yet.
It was expected that some patients would report pain located to the breast, arm, or shoulder at the reconstructed side 2-year after BR. In the present study, the one-stage group reported less pain (19% and 14%) than the two-stage group (32% and 32%), although not significantly different. It has previously been shown that up to 20% of patients report persistent pain after breast cancer treatment (PPBCT) located to the mastectomy scar or area of the missing breast (21). It has been suggested that BR increases the risk of chronic pain, but Klit et al. found no increased risk of persistent pain in patients having a reconstruction with an implant compared with mastectomy alone (odds ratio: 0.82, P=0.33) (22). A recent meta-analysis confirmed this observation as there was no significant difference between the mean prevalence of surgically related chronic pain after mastectomy alone (35.6%) or after autologous or implant-based BR (32.8%; P=0.88) (23). In the present study most of the patients in both treatment groups felt burdened (although mildly burdened) by sensory disturbance located to the field of surgery and fewer felt burdened of sensory disturbances located to the arm or shoulder on the operated side. Despite pain and sensory disturbances, all patients in the one-stage group were able to use the arm at 24-month follow-up as before surgery compared to 82% in the two-stage group. None of the patients in the one-stage group, but two patients in the two-stage group underwent axillary dissection which is associated with upper limb morbidity (24). The two patients unfortunately developed lymphedema and were burdened by this to a varying degree. All patients were offered early instruction by physiotherapist and began mobilization of the upper limb after a standardized instruction for breast reconstructive patients. Early mobilization and rehabilitation have been shown to play a significant role in reducing postoperative morbidity of the upper limb (25).
In the present study patients reported a good body image (low BIS score) in both treatment groups at both 12- and 24-month follow-up (BIS: 5.6; range, 0–30). Body image score was lower (better body image) than previously reported for immediate unilateral two-stage BR by our institution, with a mean follow-up time at 3.8 years (16.4, SD: 7.3) (18) but comparable with those found 1 year postoperatively for prophylactic mastectomies with BR (26). Atisha et al. observed a persistent good body image for immediate breast reconstructive patients from preoperatively to 2 years postoperatively suggesting that these women seem to have been “protected” from the body image disturbances normally associated with mastectomy (27).
All patients (not lost to follow-up due patient wish or explantation) thought that BR was the right choice for them and would recommend BR to others in the same situation. This is in accordance with other studies with the same study populations (18,28). As the BR was successful for the answering patients, they are supposed to be more likely to answer in a positive way compared to those with an unsuccessful or complicated BR treatment course.
In both treatment groups an increased ratio of patients (from 12- to 24-month follow-up) reported improved health status and QoL compared to the time before BR. In the present study no baseline measurement of health status or QoL was obtained and the design of the two questions may be perceived as a then-test (baseline retrospective measurement) to capture changes in internal standards and adjust for response shift (29). The patient’s assessment of an improved health state and QoL may reflect surviving a potentially life-threatening disease as breast cancer or a risk reduction. Thus, the improved QoL and general health may not be ascribed to the breast reconstructive procedure per se.
Despite limitations of this study it is strengthened by the fact that the same team of three plastic surgeons and four breast oncology surgeons performed the surgeries with standardized procedure and technique.
One-stage implant-based BR may entail advantages for the patient, but other, potentially more cost-effective, methods to obtain this has been suggested. The use of autologous dermal flaps to cover the inferior part of the implant in a similar manner than ADM, has been used for immediate one-stage BR of medium and large ptotic breasts (30) making it possibly to reduce costs compared to one-stage BR with the use of ADM (31). Although literature suggest that the risk for short-term complications is not higher than for other forms of implant-based BR, the evidence level for risk of long-term complications such as capsular contracture or PROMs and aesthetic outcome measures compared to other forms of implant-based BR is very limited (32). In 2019, Potter et al. found no statistically significant difference between complication rates of implant-based BR with biological mesh, dermal sling or synthetic mesh (33) and synthetic meshes might be a cost-effective alternative to ADM. It has been suggested that meshes as TiLOOP® and TIGR® Matrix Surgical Mesh are safe, in terms on complications, and without any difference in long-term health-related QoL and patient satisfaction in use for one-stage BR compared to BR with the use of biological mesh (34,35). A way to decrease the direct costs of ADM is meshing of the product. This technique has been investigated in a retrospective study by Scheflan et al. They found significantly shorter time to drain removal and no difference in complication rates between the two approaches with the use of meshed ADM (36).
In summary, the one-stage approach carries a shorter duration of surgery and in addition reduces the need for outpatient visits (for in average 6 times of expansion) and expander to implant exchange. In favor of the two-stage approach was reduced cost of materials due to the use of ADM in the one-stage group and fewer interventions to address the aesthetic outcome. However, pain, sensory disturbances, physical limitations, health status, QoL and body image were equally favorable between the two groups at 2-year follow-up.
Conclusions
This study does not provide clear evidence for an advantageous use of resources by one method versus the other even though the one-stage approach makes it possible to avoid outpatient visits for expansions and thereby add value for the patients. Further studies should be undertaken to investigate the cost-effectiveness of one-stage BR with ADM or with synthetic meshes in comparison with the two-stage approach. Considering the equally good results in the two treatment groups regarding PROs the one-stage approach may be preferred if the patient is deemed suitable and is well informed of the potential need for additional interventions to obtain an aesthetically satisfying result.
Acknowledgments
The authors wish to express their gratitude to the participating patients.
Funding: This work was supported by the Faculty of Health Science, Aarhus University; the Danish Cancer Society (R130-A8304-15-S38); the Korning foundation; Foundation of the Kjaersgaard Family, Sunds; King Christian X foundation; Foundation of architect Holger Hjortenberg and LifeCell Corporation (Branchburg, NJ, USA). Furthermore, LifeCell Corporation LifeCell Corporation (Branchburg, NJ, USA) provided ADM (StratticeTM) for the study.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Breast Surgery for the series “Breast Reconstruction—The True Multidisciplinary Approach”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-81/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-81/dss
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-81/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-81/coif). The series “Breast Reconstruction—The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. TED served as the unpaid Guest Editor of the series. MEB reports grants from the Danish Cancer Society, grants from the Korning foundation, grants from Foundation of the Kjaersgaard Family, Sunds, grants from King Christian X foundation, grants from Foundation of Architect Holger Hjortenberg, grants and non-financial support from LifeCell Corporation (Branchburg, NJ, USA), during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Ethics Committee of the Central Region of Denmark (1-10-72-572-12) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neuburger J, Macneill F, Jeevan R, et al. Trends in the use of bilateral mastectomy in England from 2002 to 2011: retrospective analysis of hospital episode statistics. BMJ Open 2013;3:003179. [Crossref] [PubMed]
- Fitzpatrick AM, Gao LL, Smith BL, et al. Cost and outcome analysis of breast reconstruction paradigm shift. Ann Plast Surg 2014;73:141-9. [Crossref] [PubMed]
- Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9. [Crossref] [PubMed]
- Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg 2006;57:1-5. [Crossref] [PubMed]
- Bindingnavele V, Gaon M, Ota KS, et al. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg 2007;60:1214-8. [Crossref] [PubMed]
- Johnson RK, Wright CK, Gandhi A, et al. Cost minimisation analysis of using acellular dermal matrix (Strattice™) for breast reconstruction compared with standard techniques. Eur J Surg Oncol 2013;39:242-7. [Crossref] [PubMed]
- de Blacam C, Momoh AO, Colakoglu S, et al. Cost analysis of implant-based breast reconstruction with acellular dermal matrix. Ann Plast Surg 2012;69:516-20. [Crossref] [PubMed]
- Negenborn VL, Smit JM, Dikmans REG, et al. Short-term cost-effectiveness of one-stage implant-based breast reconstruction with an acellular dermal matrix versus two-stage expander-implant reconstruction from a multicentre randomized clinical trial. Br J Surg 2019;106:586-95. [Crossref] [PubMed]
- Namnoum JD. Expander/implant reconstruction with AlloDerm: recent experience. Plast Reconstr Surg 2009;124:387-94. [Crossref] [PubMed]
- Breuing KH, Colwell AS. Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg 2007;59:250-5. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Berry C, et al. Acellular dermal matrix-assisted direct-to-implant breast reconstruction and capsular contracture: a 13-year experience. Plast Reconstr Surg 2016;138:329-37. [Crossref] [PubMed]
- Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg 2011;128:403e-10e. [Crossref] [PubMed]
- Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 2010;125:429-36. [Crossref] [PubMed]
- Hansson E, Edvinsson AC, Hallberg H. Drain secretion and seroma formation after immediate breast reconstruction with a biological and a synthetic mesh, respectively: A randomized controlled study. Breast J 2020;26:1756-9. [Crossref] [PubMed]
- Brunbjerg ME, Jensen TB, Overgaard J, et al. Comparison of one-stage direct-to-implant with acellular dermal matrix and two-stage immediate implant-based breast reconstruction-a cohort study. Gland Surg 2021;10:207-18. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24. [Crossref] [PubMed]
- Hopwood P, Fletcher I, Lee A, et al. A body image scale for use with cancer patients. Eur J Cancer 2001;37:189-97. [Crossref] [PubMed]
- Juhl AA, Christensen S, Zachariae R, et al. Unilateral breast reconstruction after mastectomy - patient satisfaction, aesthetic outcome and quality of life. Acta Oncol 2017;56:225-31. [Crossref] [PubMed]
- Krishnan NM, Fischer JP, Basta MN, et al. Is single-stage prosthetic reconstruction cost effective? A cost-utility analysis for the use of direct-to-implant breast reconstruction relative to expander-implant reconstruction in postmastectomy patients. Plast Reconstr Surg 2016;138:537-47. [Crossref] [PubMed]
- Susarla SM, Ganske I, Helliwell L, et al. Comparison of clinical outcomes and patient satisfaction in immediate single-stage versus two-stage implant-based breast reconstruction. Plast Reconstr Surg 2015;135:1e-8e. [Crossref] [PubMed]
- Juhl AA, Christiansen P, Damsgaard TE. Persistent pain after breast cancer treatment: a questionnaire-based study on the prevalence, associated treatment variables, and pain type. J Breast Cancer 2016;19:447-54. [Crossref] [PubMed]
- Klit A, Mejdahl MK, Gärtner R, et al. Breast reconstruction with an expander prosthesis following mastectomy does not cause additional persistent pain: a nationwide cross-sectional study. J Plast Reconstr Aesthet Surg 2013;66:1652-8. [Crossref] [PubMed]
- Reghunathan M, Rahgozar P, Sbitany H, et al. Breast reconstruction does not increase the incidence of postmastectomy pain syndrome: results of a meta-analysis. Ann Plast Surg 2020;84:611-7. [Crossref] [PubMed]
- Sagen A, Kaaresen R, Sandvik L, et al. Upper limb physical function and adverse effects after breast cancer surgery: a prospective 2.5-year follow-up study and preoperative measures. Arch Phys Med Rehabil 2014;95:875-81. [Crossref] [PubMed]
- Scaffidi M, Vulpiani MC, Vetrano M, et al. Early rehabilitation reduces the onset of complications in the upper limb following breast cancer surgery. Eur J Phys Rehabil Med 2012;48:601-11. [PubMed]
- Hopwood P, Lee A, Shenton A, et al. Clinical follow-up after bilateral risk reducing ('prophylactic') mastectomy: mental health and body image outcomes. Psychooncology 2000;9:462-72. [Crossref] [PubMed]
- Atisha D, Alderman AK, Lowery JC, et al. Prospective analysis of long-term psychosocial outcomes in breast reconstruction: two-year postoperative results from the Michigan Breast Reconstruction Outcomes Study. Ann Surg 2008;247:1019-28. [Crossref] [PubMed]
- Apte A, Walsh M, Chandrasekharan S, et al. Single-stage immediate breast reconstruction with acellular dermal matrix: experience gained and lessons learnt from patient reported outcome measures. Eur J Surg Oncol 2016;42:39-44. [Crossref] [PubMed]
- Hamidou Z, Dabakuyo TS, Bonnetain F. Impact of response shift on longitudinal quality-of-life assessment in cancer clinical trials. Expert Rev Pharmacoecon Outcomes Res 2011;11:549-59. [Crossref] [PubMed]
- Nava MB, Cortinovis U, Ottolenghi J, et al. Skin-reducing mastectomy. Plast Reconstr Surg 2006;118:603-10; discussion 611-3. [Crossref] [PubMed]
- Krishnan NM, Chatterjee A, Van Vliet MM, et al. A comparison of acellular dermal matrix to autologous dermal flaps in single-stage, implant-based immediate breast reconstruction: a cost-effectiveness analysis. Plast Reconstr Surg 2013;131:953-61. [Crossref] [PubMed]
- Jepsen C, Hallberg H, Pivodic A, et al. Complications, patient-reported outcomes, and aesthetic results in immediate breast reconstruction with a dermal sling: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2019;72:369-80. [Crossref] [PubMed]
- Potter S, Conroy EJ, Cutress RI, et al. Short-term safety outcomes of mastectomy and immediate implant-based breast reconstruction with and without mesh (iBRA): a multicentre, prospective cohort study. Lancet Oncol 2019;20:254-66. [Crossref] [PubMed]
- Hallberg H, Elander A, Kölby L, et al. A biological or a synthetic mesh in immediate breast reconstruction? A cohort-study of long-term Health related Quality of Life (HrQoL). Eur J Surg Oncol 2019;45:1812-6. [Crossref] [PubMed]
- Gschwantler-Kaulich D, Schrenk P, Bjelic-Radisic V, et al. Mesh versus acellular dermal matrix in immediate implant-based breast reconstruction - A prospective randomized trial. Eur J Surg Oncol 2016;42:665-71. [Crossref] [PubMed]
- Scheflan M, Allweis TM, Ben Yehuda D, et al. Meshed acellular dermal matrix in immediate prepectoral implant-based breast reconstruction. Plast Reconstr Surg Glob Open 2020;8:e3265. [Crossref] [PubMed]
Cite this article as: Brunbjerg ME, Jensen TB, Overgaard J, Christiansen P, Damsgaard TE. Resource utilization and patient reported outcomes using acellular dermal matrix in breast reconstructive procedures. Ann Breast Surg 2023;7:3.

