
An 11-year retrospective analysis of clinical outcomes after prepectoral implant-based breast reconstruction performed by a single surgeon
Introduction
The most common method of breast reconstruction offered to breast cancer patients following mastectomy is implant-based breast reconstruction (1). Traditionally, prosthetic breast reconstruction has been performed in the submuscular plane and has evolved over time from complete coverage of the implant with the pectoralis and serratus muscles and fascia to partial submuscular coverage with the use of acellular dermal matrix (ADM) to cover the lower pole (2-8). Prepectoral implant breast reconstruction has become an alternative reconstructive option to partial and total submuscular coverage methods and can incorporate total implant coverage with ADM, or a combination of upper pole coverage with an ADM sling and lower pole coverage with an inferior de-epithelialized dermal flap (9). The placement of an implant or tissue expander in the prepectoral space with the aid of ADM avoids elevation of the pectoralis and serratus muscles and fascia. It is a muscle-sparing procedure, resulting in less postoperative pain, faster recovery and lower risk of animation deformity compared to subpectoral reconstruction (10,11). Multiple studies have shown acceptable outcomes after prepectoral breast reconstruction compared to submuscular reconstruction (12-21).
Despite these advantages, broad acceptance of the procedure is still slow secondary to safety concerns, including a fear of increased risk of capsular contracture, implant exposure and implant visibility, as well as delayed detection of breast cancer recurrence. This study aimed to describe clinical outcomes in prepectoral breast reconstruction performed by a single surgeon over an 11-year period, and to compare outcomes after prepectoral reconstruction to outcomes after subpectoral reconstruction performed by the same surgeon during the same study period. We present the following article in accordance with the STROBE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-78/rc).
Methods
A retrospective chart review was performed of all patients who had prepectoral or subpectoral implant-based breast reconstruction with an inferior de-epithelialized dermal flap and ADM performed by the senior author (A.O.Y) at Mount Sinai South Nassau in Oceanside, New York and Yale New Haven Health/Bridgeport Hospital in Bridgeport, Connecticut between January 1st, 2010 and April 30th, 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the NYU Winthrop Institutional Review Board (IRB approval number 17411) and informed consent for this retrospective analysis was waived.
Demographic characteristics were evaluated, including age, body mass index (BMI), active smoking, history of diabetes and radiation exposure. Clinical and operative characteristics were also recorded, including laterality, prophylactic or therapeutic indication, implant volume, whether autologous fat grafting was performed as a secondary procedure, and whether the nipple-areolar complex was harvested and repositioned as a free nipple graft. Outcomes were assessed by calculating complication rates, including capsular contracture, infection, mastectomy skin flap necrosis (MSFN), implant loss, seroma, hematoma, dehiscence and local recurrence. Major complications were defined as those requiring rehospitalization or reoperation, and minor complications were managed without rehospitalization or reoperation.
All patients who had prepectoral or subpectoral implant breast reconstruction with an inferior de-epithelialized dermal flap with a Wise pattern or modified-Wise pattern mastectomy incision and ADM were included. Patients who underwent prepectoral or subpectoral implant breast reconstruction with other mastectomy incision patterns were excluded. Patients with a history of premastectomy radiation therapy who underwent prepectoral or subpectoral implant breast reconstruction were also excluded because implant breast reconstruction in this group of patients is not standard of care. The majority of patients in the study underwent Wise pattern mastectomy and were large breasted, had a certain degree of ptosis and wanted to be smaller or similar in breast size. Of these patients, those who were suitable candidates and for whom it was oncologically safe, had the nipple-areolar complex harvested as a full-thickness graft and grafted to a new location on the reconstructed breast. Patients who did not have the nipple-areolar complex harvested as a free graft had the nipple sacrificed with the Wise pattern mastectomy. A small number of patients in the study who did not have ptosis underwent nipple-sparing mastectomy with the nipple-areolar complex left intact and implant reconstruction. Postoperatively, patients were seen in clinic weekly or bi-weekly, or until it was confirmed that complete wound healing had occurred without complications.
Materials
Smooth round silicone gel implants or expandable saline implants (Mentor Worldwide LLC, Irvine, CA, USA) and porcine-derived Strattice® acellular dermal matrix (Allergan Inc, Irvine, CA, USA) were used in all reconstructions.
Prepectoral implant breast reconstruction technique
As most patients had some degree of ptosis, a Wise or modified-Wise pattern mastectomy incision with an inferior de-epithelialized dermal flap was used (Figure 1A-1F). To create the implant pocket, a piece of ADM was sewn to the medial point of the mastectomy pocket, the superior point of the pectoralis muscle and the superior point of the inferior de-epithelialized dermal flap. The implant was covered with ADM superiorly and the de-epithelialized dermal flap inferiorly and was sutured down to the chest wall, serratus fascia and inferior de-epithelialized dermal flap, closing the implant pocket. One closed suction drain was placed in the mastectomy pocket and was removed after 2 weeks in non-radiated breasts and after 3 to 4 weeks in radiated breasts. Patients for whom it was oncologically safe, had the nipple-areolar complex harvested as a full-thickness free nipple graft and wrapped in saline-moistened gauze. After mastectomy and implant reconstruction was performed, the skin overlying the ideal nipple location on the breast mound was de-epithelialized and the free graft was sutured in place (Figures 2,3). The vast majority of reconstructions were performed in a single-stage direct-to-implant approach. Patients who had a deficit of skin and who wished to accomplish a significant increase in breast size were accommodated with a two-stage expander-to-implant reconstruction. In this study, autologous fat grafting was an integral component of breast reconstruction as a way to improve contour deformities and customize the upper and medial pole.

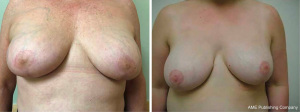
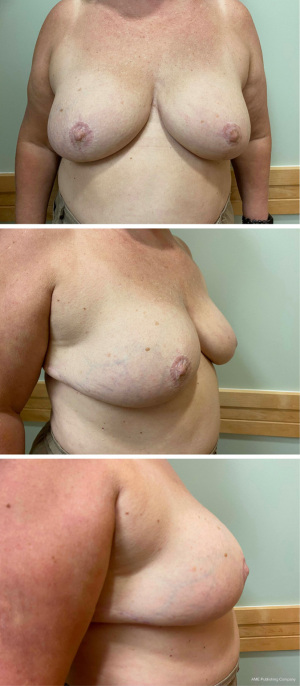
Subpectoral implant reconstruction was performed in a similar manner using a Wise or modified-Wise mastectomy incision. An inferior de-epithelialized dermal flap was fashioned from excess lower-pole breast tissue. The pectoralis major muscle was elevated off the chest wall. The superior aspect of the de-epithelialized dermal flap was sewn to the inferior aspect of the elevated pectoralis major muscle to create the implant pocket, and a lateral piece of ADM was sewn to the inferior de-epithelialized dermal flap, the pectoralis major muscle and the lateral chest wall fascia. After the implant was placed in the mastectomy implant pocket, the implant pocket was closed by placing lateral sutures in the ADM.
Statistical analysis
Continuous variables were described as the mean ± SD and compared using a two-tailed t-test. Categorical variables were described as percentages. To determine associations between categorical variables and complication rates, a two-tailed Fisher’s exact test was used. P values less than 0.05 were considered significant.
Results
Over the 11-year study period, 758 prepectoral implant breast reconstructions were performed in 468 patients with an average follow-up of 24 months, a mean age of 52.5±9.9 (± SD) years and mean body mass index (BMI) of 28.8±6.1 kg/m2 (Table 1). Twenty-seven patients (5.8%) were active smokers, 24 patients (5.1%) were diabetic and 107 breasts (14.1%) received postmastectomy radiation therapy. The majority of reconstructions were bilateral (62.0%) and therapeutic (54.5%) and 98.3% were single-stage direct-to-implant. Mean implant volume was 356.0±123.8 cc, 41.3% had autologous fat grafting for contour deformities as a secondary procedure and 52.6% had a nipple-areolar complex free graft.
Table 1
| Characteristics | Prepectoral total | Subpectoral total | P value |
|---|---|---|---|
| No. of patients | 468 | 100 | |
| No. of breasts | 758 | 163 | |
| Follow-up‡ (months) | 23.6±24.0 | 31.9±22.4 | 0.0016* |
| Demographic | |||
| Age‡ (years) | 52.5±9.9 | 46.9±8.8 | 0.0001* |
| BMI‡ (kg/m2) | 28.8±6.1 | 25.2±5.0 | 0.0001* |
| Smokers | 5.8 [27] | 5.0 [5] | 1.0000 |
| Diabetes | 5.1 [24] | 3.0 [3] | 0.4487 |
| Postmastectomy radiation | 14.1 [107] | 14.1 [23] | 1.0000 |
| Clinical | |||
| Unilateral | 38.0 [178] | 37.0 [37] | 0.9097 |
| Bilateral | 62.0 [290] | 63.0 [63] | |
| Prophylactic | 45.5 [345] | 37.4 [61] | 0.0677 |
| Therapeutic | 54.5 [413] | 62.6 [102] | |
| Single-stage | 98.3 [745] | 72.4 [118] | 0.0001* |
| Two-stage | 1.7 [13] | 27.6 [45] | |
| Implant volume (cc) | 356.0±123.8 | 366.1±136.8 | 0.3541 |
| Autologous fat grafting | 41.3 [313] | 77.9 [127] | 0.0001* |
| Free nipple graft | 52.6 [399] | 33.1 [54] | 0.0001* |
| Complications | |||
| Infection (major) | 3.4 [26] | 1.2 [2] | 0.2056 |
| Infection (minor) | 0.9 [7] | 0.6 [1] | 1.0000 |
| Seroma | 0.3 [2] | 1.2 [2] | 0.1462 |
| Hematoma | 0.3 [2] | 0 | 1.0000 |
| Dehiscence | 0.7 [5] | 1.2 [2] | 0.3593 |
| Necrosis (major) | 1.7 [13] | 1.2 [2] | 1.0000 |
| Necrosis (minor) | 3.4 [26] | 1.2 [2] | 0.2056 |
| Capsular contracture | 6.5 [50] | 9.8 [16] | 0.1786 |
| Implant loss | 4.1 [31] | 4.3 [7] | 0.8305 |
| Local recurrence total | 1.3 [10] | 1.2 [2] | 1.0000 |
| Local recurrence therapeutic | 2.4 [10] | 1.9 [2] | 1.0000 |
| Complications total | 22.7 [172] | 22.1 [36] | 0.9180 |
| ≥1 complication | 14.8 [113] | 16.6 [27] | 0.4689 |
‡, continuous variables reported as the mean ± SD. Categorical variables reported as percentages with the number comprising the percentages in brackets. *, the difference is statistically significant. Adapted from Ann Surg Oncol 2018;25(10):2899-2908. BMI, body mass index; SD, standard deviation.
During the same study period, 163 subpectoral implant breast reconstructions were performed by the same surgeon in 100 patients with a mean follow-up of 32 months, a mean age of 46.9±8.8 years and a mean BMI of 25.2±5.0 kg/m2. Five patients (5.0%) were active smokers, 3 patients (3.0%) were diabetic and 23 breasts (14.1%) received postmastectomy radiation therapy. Similar to the prepectoral cohort, the majority of reconstructions were bilateral (63.0%) and therapeutic (62.6%) and 72.4% were single-stage direct-to-implant. Mean implant volume was 366.1±136.8 cc, 77.9% had autologous fat grafting as a secondary procedure and 33.1% had a nipple-areolar complex free graft.
Demographic and clinical characteristics were similar between prepectoral and subpectoral implant reconstruction patients; however, prepectoral reconstruction patients were older (52.5±9.9 vs. 46.9±8.8 years; P=0.0001), had a higher BMI (28.8±6.1 vs. 25.2±5.0 kg/m2; P=0.0001), a shorter follow-up period (23.6±24.0 vs. 31.9±22.4 months; P=0.0016), a larger percentage of free nipple grafts (52.6% vs. 33.1%), a smaller percentage of autologous fat grafting (41.6% vs. 77.9%) and a larger percentage of single-stage reconstructions (98.3% vs. 72.4%), respectively (P=0.0001). The higher BMI in prepectoral reconstruction patients can be attributed to the need for more upper-pole soft-tissue coverage required to perform this technique compared to subpectoral implant reconstruction. The shorter follow-up period in prepectoral reconstruction patients can be attributed to the fact that this technique was adopted and incorporated into the reconstructive armamentarium of the senior author later in her career.
Complication rates were low after prepectoral implant reconstruction, with capsular contracture (6.5%), implant loss (4.1%), major infection and minor mastectomy skin flap necrosis (3.4%) being the most common. Complication rates after prepectoral implant reconstruction were comparable to those after subpectoral implant reconstruction, with regard to major infection (3.4% vs. 1.2%), minor infection (0.9% vs. 0.6%), seroma (0.3% vs. 1.2%), hematoma (0.3% vs. 0%), dehiscence (0.7% vs. 1.2%), major mastectomy skin flap necrosis (1.7% vs. 1.2%), minor mastectomy skin flap necrosis (3.4% vs. 1.2%), capsular contracture (6.5% vs. 9.8%), implant loss (4.1% vs. 4.3%), local recurrence (1.3% vs. 1.2%), local recurrence in therapeutic reconstructions (2.4% vs. 1.9%), total complications (22.7% vs. 22.1%) and breasts with one or more complication (14.8% vs. 16.6%), respectively (P≥0.1462). It is important to note that in many cases multiple complications occurred in the same breast, as poor mastectomy skin flap quality often leads to necrosis, infection, seroma and implant loss.
To determine risk factors for complications after prepectoral implant reconstruction, demographic, clinical and operative characteristics were compared between patients with one or more major or minor complications to patients without a complication (Table 2). Demographic, clinical and operative characteristics were similar in patients with a complication to patients without a complication; however, patients with a complication had a higher rate of receiving postmastectomy radiation therapy (28.0% vs. 11.6%; P=0.0001), a higher rate of therapeutic reconstruction (64.4% vs. 52.6%; P=0.0206) and a longer follow-up period (35.5±29.0 vs. 22.3±22.8 months; P=0.0001). Age, BMI, active smoking, diabetes, bilateral reconstruction, single-stage reconstruction, implant volume and autologous fat grafting were not significantly associated with a complication after prepectoral implant reconstruction.
Table 2
| Characteristics | ≥1 complication | No complication | P value |
|---|---|---|---|
| No. of patients | 104 | 424 | |
| No. of breasts | 118 | 640 | |
| Follow-up‡ (months) | 35.5±29.0 | 22.3±22.8 | 0.0001* |
| Demographic | |||
| Age‡ (years) | 51.6±9.3 | 52.4±9.9 | 0.4553 |
| BMI‡ (kg/m2) | 29.5±6.9 | 28.7±5.8 | 0.2260 |
| Smokers | 8.6 [9] | 5.4 [23] | 0.2494 |
| Diabetes | 8.6 [9] | 3.8 [16] | 0.0659 |
| Postmastectomy radiation | 28.0 [33] | 11.6 [74] | 0.0001* |
| Operative and clinical | |||
| Unilateral | 30.8 [32] | 34.4 [146] | 0.5629 |
| Bilateral | 69.2 [72] | 65.6 [278] | 0.5629 |
| Prophylactic | 35.6 [42] | 47.3 [303] | 0.0206* |
| Therapeutic | 64.4 [76] | 52.6 [337] | 0.0206* |
| Single-stage | 97.4 [115] | 98.4 [630] | 0.4381 |
| Two-stage | 2.5 [3] | 1.6 [10] | 0.4381 |
| Implant volume (cc) | 370.7±129.1 | 353.3±122.7 | 0.1608 |
| Autologous fat grafting | 43.2 [51] | 40.9 [262] | 0.6843 |
‡, continuous variables reported as the mean ± SD. Categorical variables reported as percentages with the number comprising the percentages in brackets. *, the difference is statistically significant. BMI, body mass index; SD, standard deviation.
Discussion
Broad acceptance of prepectoral implant-based breast reconstruction as an alternative to submuscular implant reconstruction after mastectomy has been slow secondary to a fear of increased risk of capsular contracture, implant exposure and implant visibility, as well as delayed detection of breast cancer recurrence. The results of this large cohort study of 758 prepectoral implant-based reconstructions in 468 patients performed by single surgeon over an 11-year period show that this muscle-sparing technique of direct-to-implant reconstruction can be performed safely with low complication rates, comparable to those associated with subpectoral implant reconstruction. Rates of capsular contracture, implant exposure and local recurrence were not increased in this study during the senior author’s 11-year experience performing prepectoral implant breast reconstruction. Furthermore, postmastectomy radiation therapy and therapeutic reconstruction were risk factors for a major or minor complication after prepectoral implant reconstruction. Another important outcome of this study is that it demonstrates that larger breasted women with ptosis who are not ideal candidates for nipple-sparing mastectomy can safely undergo immediate prepectoral implant reconstruction using a Wise pattern mastectomy incision with the nipple-areolar complex harvested and repositioned in an ideal location as a free graft.
In a large 12-year study of 1,522 two-stage expander-to-implant subpectoral reconstructions in 1,221 patients, Cordeiro and McCarthy reported outcomes comparable to that of our study. The authors reported an overall early total complication rate of 5.8%, with the most common complication being infection (2.5%), followed by native skin flap necrosis (2.0%), hematoma (0.4%), seroma (0.2%) and delayed wound healing (0.4%) (2). In a follow-up study, the authors reported long-term complications in a subgroup of 410 reconstructions in 315 patients with a minimum of 1 year of follow-up (22). The rates of severe Grade III and IV capsular contracture in the group as a whole was 18.1%, which included patients who received pre- and postmastectomy radiation and was 10.3% in non-radiated patients. These rates of capsular contracture were higher than that reported in our study (6.5%) which included Baker’s Grade II through IV capsular contracture.
The results of this large study also compare favorably with other published studies on clinical outcomes after prepectoral implant breast reconstruction. In a study comparing prepectoral breast reconstruction in 51 patients (84 breasts) to subpectoral breast reconstruction in 115 patients (186 breasts), Sbitany et al. showed the overall total complication rate was 17.9% in the prepectoral group and was comparable to that of the subpectoral group (18.8%) (16). In the prepectoral group the most common complications were minor infection (4.8%), seroma (3.6%), major infection and hematoma (2.4%) and major mastectomy skin flap necrosis and implant loss (1.2%). In the subpectoral group, the most common complications were minor infection (9.1%), seroma (6.5%), major infection (5.9%), implant loss and major mastectomy skin flap necrosis (4.3%) and hematoma (1.1%). In this study, complications in both prepectoral and subpectoral patients compared favorably with that of our study. Nealon et al. reported an overall complication of 14.0% in 183 prepectoral direct-to-implant reconstructions (114 patients), with capsular contracture comprising 1.8%, major MSFN 4.4%, seroma 8.8%, hematoma 5.3% and implant loss 3.5% (23). In this study, 6.1% of prepectoral reconstructions received premastectomy radiation therapy and 24.6% received postmastectomy radiation therapy. Interestingly, rates of capsular contracture (1.8% vs. 6.5%) and implant loss (3.5% vs. 4.1%) in this study were lower than that reported in our study, while rates of major MSFN (4.4% vs. 1.7%), seroma (8.8% vs. 0.3%) and hematoma (5.3% vs. 0.3%) were higher.
The non-randomized, retrospective design are limitations of this study because of the potential for selection bias. However, the significance of this study lies in that it demonstrates that prepectoral breast reconstruction can be performed safely and effectively with complication rates comparable to that of submuscular reconstructive methods. In settings where there is an experienced team of oncoplastic surgeons, prepectoral breast reconstruction should be offered to breast cancer patients after mastectomy because of low complication rates, reduced invasiveness of the procedure, postoperative pain and faster recovery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-78/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-78/dss
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-78/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-78/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the NYU Winthrop Institutional Review Board (IRB approval number 17411) and informed consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Society of Plastic Surgeons, Plastic surgery statistics report 2019. Available online: https://www.plasticsurgery.org/news/plastic-surgery-statistics. Accessed December 10th, 2020.
- Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications. Plast Reconstr Surg 2006;118:825-31. [Crossref] [PubMed]
- Sorkin M, Qi J, Kim HM, et al. Acellular Dermal Matrix in Immediate Expander/Implant Breast Reconstruction: A Multicenter Assessment of Risks and Benefits. Plast Reconstr Surg 2017;140:1091-100. [Crossref] [PubMed]
- Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg 2009;124:1735-40. [Crossref] [PubMed]
- Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg 2011;128:403e-10e. [Crossref] [PubMed]
- Salzberg CA. Focus on technique: one-stage implant-based breast reconstruction. Plast Reconstr Surg 2012;130:95S-103S. [Crossref] [PubMed]
- Salzberg CA, Dunavant C, Nocera N. Immediate breast reconstruction using porcine acellular dermal matrix (Strattice™): long-term outcomes and complications. J Plast Reconstr Aesthet Surg 2013;66:323-8. [Crossref] [PubMed]
- Colwell AS, Christensen JM. Nipple-Sparing Mastectomy and Direct-to-Implant Breast Reconstruction. Plast Reconstr Surg 2017;140:44S-50S. [Crossref] [PubMed]
- Sinnott CJ, Persing SM, Pronovost M, et al. Impact of Postmastectomy Radiation Therapy in Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction. Ann Surg Oncol 2018;25:2899-908. [Crossref] [PubMed]
- Kobraei EM, Cauley R, Gadd M, et al. Avoiding Breast Animation Deformity with Pectoralis-Sparing Subcutaneous Direct-to-Implant Breast Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e708. [Crossref] [PubMed]
- Spear SL, Schwartz J, Dayan JH, et al. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg 2009;33:44-8. [Crossref] [PubMed]
- Bettinger LN, Waters LM, Reese SW, et al. Comparative Study of Prepectoral and Subpectoral Expander-Based Breast Reconstruction and Clavien IIIb Score Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1433. [Crossref] [PubMed]
- Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg 2016;69:e77-86. [Crossref] [PubMed]
- Downs RK, Hedges K. An Alternative Technique for Immediate Direct-to-Implant Breast Reconstruction-A Case Series. Plast Reconstr Surg Glob Open 2016;4:e821. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Sbitany H, Piper M, Lentz R. Prepectoral Breast Reconstruction: A Safe Alternative to Submuscular Prosthetic Reconstruction following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;140:432-43. [Crossref] [PubMed]
- Caputo GG, Marchetti A, Dalla Pozza E, et al. Skin-Reduction Breast Reconstructions with Prepectoral Implant. Plast Reconstr Surg 2016;137:1702-5. [Crossref] [PubMed]
- Onesti MG, Maruccia M, Di Taranto G, et al. Clinical, histological, and ultrasound follow-up of breast reconstruction with one-stage muscle-sparing "wrap" technique: A single-center experience. J Plast Reconstr Aesthet Surg 2017;70:1527-36. [Crossref] [PubMed]
- Avila A, Bartholomew AJ, Sosin M, et al. Acute Postoperative Complications in Prepectoral versus Subpectoral Reconstruction following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2020;146:715e-20e. [Crossref] [PubMed]
- Ricci JA, Epstein S, Momoh AO, et al. A meta-analysis of implant-based breast reconstruction and timing of adjuvant radiation therapy. J Surg Res 2017;218:108-16. [Crossref] [PubMed]
- Magill LJ, Robertson FP, Jell G, et al. Determining the outcomes of post-mastectomy radiation therapy delivered to the definitive implant in patients undergoing one- and two-stage implant-based breast reconstruction: A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2017;70:1329-35. [Crossref] [PubMed]
- Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plast Reconstr Surg 2006;118:832-9. [Crossref] [PubMed]
- Nealon KP, Weitzman RE, Sobti N, et al. Prepectoral Direct-to-Implant Breast Reconstruction: Safety Outcome Endpoints and Delineation of Risk Factors. Plast Reconstr Surg 2020;145:898e-908e. [Crossref] [PubMed]
Cite this article as: Sinnott CJ, Pronovost MT, Hodyl C, Lynch M, Young F, Edwards S, Young AO. An 11-year retrospective analysis of clinical outcomes after prepectoral implant-based breast reconstruction performed by a single surgeon. Ann Breast Surg 2022;6:31.

