
Investigating the time to adjuvant treatment following immediate breast reconstruction in breast cancer patients
Introduction
Breast cancer surgery has undergone major advances in the past two decades (1). Breast conserving surgery has become the surgery of choice instead of mastectomy for the great majority of patients. However, mastectomy remains the appropriate surgical treatment for up to 40% of breast cancer patients (2). Mastectomy can have a significant negative impact on quality of life with reduced self-esteem, poor body image and effect on sexuality and relationships all reported (3). Restoring patients’ body image is a crucial component of patient care and has become an integral aspect of the holistic approach to breast cancer treatment. It is possible to carry out reconstruction at the time of mastectomy: “immediate”, [post-mastectomy immediate breast reconstruction (PMIBR)] or at a later time: “delayed” (4).
The National Mastectomy and Breast Reconstruction Audit (NMBRA) (3) shows similar patient outcomes and satisfaction for immediate and delayed reconstruction for all parameters: aesthetic appearance, emotional, physical and sexual well-being.
Yet, immediate breast reconstruction is now considered as gold standard of care and all suitable patients undergoing mastectomy should be offered immediate reconstruction according to the National Institute of Clinical Excellence (NICE) guidelines (5). The two main options for performing PMIBR following a conservative mastectomy are implant-based or autologous flaps. The NMBRA has shown slightly better patient-reported satisfaction for autologous reconstructions in comparison with implants, an observation that has also been reported by others (6-8).
However, there is concern that immediate reconstruction could cause a delay in commencing adjuvant therapy as a direct consequence of an increased rate of post-operative complications caused by the added complexity of the surgical procedure (9). The optimum duration between surgery and delivery of adjuvant therapy has not yet been clearly defined. NICE guidelines suggest that subsequent or adjuvant treatment should ideally commence by 31 days following definitive surgery (5), conversely the American Society of Clinical Oncology recommends that treatment be completed within 121 days of diagnosis (10). A 2011 Cochrane review (11) reported that there was lack of evidence on either side of the debate and that local guidelines should be followed for best practice. This clearly implies that there is a paucity of data in this area and unanswered questions remain. Literatures from tertiary centres in UK are particularly lacking—making our study highly relevant to this area.
The primary aim of this study is therefore to explore the association between immediate breast reconstruction and the timing to adjuvant treatment. Immediate breast reconstruction is increasingly being offered as part of surgical treatment around the world, in line with various international guidelines. Any ‘perceived’ delay to delivering adjuvant treatment due to the increased complexity of surgery and possible post-operative complications, may cause anxiety to both patients and clinicians. Our study may assist in adding further support to current practice and recommendations. We present the following article in accordance with the STROBE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-37/rc).
Methods
Patients
This is a single centre retrospective study: all patients were treated at the Guy’s & St Thomas’ Hospitals (GSTT) Breast Unit between January 2015 and December 2018. Suitable patients were identified using the GSTT Breast Unit’s electronic theatre scheduling system. The keywords “mastectomy” and “immediate reconstruction” were used to identify patients suitable for this study. We proceeded to manually sort the records obtained into the sub-categories of: “autologous” or “implant” based immediate breast reconstruction. All patients who received adjuvant treatment (chemotherapy, radiotherapy or both) following immediate breast reconstruction, using either autologous free flaps (n=80) or implant-based (n=88) were included (total n=168). Patients undergoing risk-reducing prophylactic mastectomy or those that did not receive adjuvant treatment were excluded from this study (n=310). The control group included patients who underwent wide local excision (WLE) a common breast-conserving surgery (n=65), or mastectomy (n=20) without reconstruction— followed by adjuvant treatment as illustrated in Figure 1. The number of patients in the control group is matched 1:2 with the immediate reconstruction group. The decision to include patients undergoing breast conserving surgery was made due to the low number of patients undergoing mastectomy without immediate breast reconstruction at our centre. Control group patients were identified from the electronic database using keywords: “mastectomy”, “simple mastectomy”, “mastectomy without reconstruction” and “WLE”, data was included up to January 2019. Confidential patient information was rendered anonymous without breaching the duty of confidentiality—the database was independently established for clinical research purposes as per Health Research Authority guidelines. Thus, informed consent and Research Ethics Committee guidance was not necessary for this study. Demographic, tumour histology and receptor status is included in Table 1.
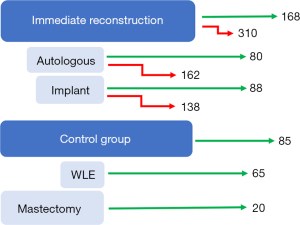
Table 1
| Category | DIEP (n=80) | Implant (n=88) | Total (n=168) | Control (n=85) |
|---|---|---|---|---|
| Age (years) | ||||
| Median | 49 | 46 | 47 | 62 |
| Mean (SD) | 48.8 (7.8) | 44.0 (10.7) | 47.4 (9.5) | 60.7 (11.8) |
| <35 | 3.8% | 13.6% | 8.9% | 4.3% |
| 36–50 | 51.3% | 53.4% | 52.4% | 12.8% |
| 51–69 | 45.0% | 30.7% | 37.5% | 61.7% |
| >70 | 0.0% | 2.3% | 1.2% | 19.1% |
| Smoking history | ||||
| Yes | 11.70% | 14.60% | 13.10% | – |
| BMI (kg/m2) | ||||
| Median | 29.3 | 25.6 | 28 | – |
| Mean (SD) | 29.1 (3.2) | 28.4 (7.8) | 28.8 (5.4) | – |
| Tumour histology | ||||
| DCIS | 3.8% | 3.4% | 3.6% | 14.6% |
| Invasive ductal/NST | 82.3% | 86.4% | 83.9% | 62.5% |
| Invasive lobular | 8.9% | 5.7% | 7.1% | 6.3% |
| Mixed | 1.3% | 2.8% | 2.4% | 4.2% |
| Others | 3.8% | 2.3% | 3.0% | 12.5% |
| Receptor status | ||||
| ER+/HER2+ | 10% | 11.4% | 10.7% | 11.9% |
| ER+/HER2− | 67.5% | 63.6% | 65.5% | 69.0% |
| ER−/HER2+ | 11.3% | 5.7% | 8.3% | 7.1% |
| Triple− | 11.3% | 19.3% | 15.5% | 11.9% |
DIEP, deep inferior epigastric perforators; SD, standard deviation; BMI, body mass index; DCIS, ductal carcinoma in situ; NST, no specific type; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2.
Assessment of outcomes and clinical covariates
Our primary outcome was the time between breast cancer surgery and first adjuvant treatment. In our study, we defined a delay to adjuvant treatment as patients receiving their first adjuvant intervention more than 90 days after their surgery. This is consistent with available literature (12), in particular a recent study with a large sample size (n=24,843) showing that time to adjuvant treatment exceeding 90 days is associated with adverse clinical outcomes: in particular, overall survival and patient-recorded outcomes. In our study, the 90-day definition was equivalent to the mean time between surgery and adjuvant treatment plus one standard deviation. Variables collected from the two groups include: date of birth, surgery date, radiotherapy date, chemotherapy date, complications, tumour size, histological characteristics, lymph node information, demographic information.
Statistical analysis
Regression analysis was undertaken to check for any association with other collected parameters: no confounding associations were found. Table 2 shows the output of our regression analysis.
Table 2
| Variables | Estimate | Standard error | t value | P value |
|---|---|---|---|---|
| Age | 0.7735 | 0.4424 | 1.749 | 0.0863 |
| BMI | 1.0538 | 0.6673 | 1.579 | 0.1204 |
| Preop ultrasound size | 0.1041 | 0.1673 | 0.622 | 0.5364 |
| ER+ve | −5.5873 | 15.0575 | −0.371 | 0.713 |
| PR+ve | −0.8083 | 14.1968 | −0.057 | 0.955 |
| HER2+ve | −7.2328 | 17.8692 | −0.405 | 0.688 |
| Smoking | −0.4115 | 16.8557 | −0.024 | 0.981 |
| Unifocal/multifocal | −10.0618 | 11.845 | −0.849 | 0.401 |
| Axillary node positive | 19.2842 | 12.9362 | 1.498 | 0.143 |
ER, oestrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; +ve, positive.
Statistical analysis was undertaken using unpaired t-tests to compare time to adjuvant treatment within the immediate reconstruction and control groups to determine if there are significant differences.
Ethical statement
This study adheres to the guidelines on medical protocols and ethics stated in the Declaration of Helsinki (as revised in 2013). The authors assert that ethical approval for publication of this manuscript was not required by their local Ethics Committee.
Results
Our results show similar mean time to adjuvant treatment between the two groups: 65.4 days (95% CI ±3.8 days) for the immediate reconstruction group and 65.3 days (95% CI ±5.1 days) for the control group (P=0.988). Furthermore, within the immediate breast reconstruction group, the time to adjuvant treatment was 65.3 days (95% CI ±6.2 days) for the implant group and 65.5 days (95% CI ±5.47 days) for the autologous group (P=0.964), as illustrated in Figure 2. We identified a delay to adjuvant treatment in 20 patients out of the 168 that underwent immediate breast reconstruction (11.9%); the causes for delay by type are explored in the discussion.
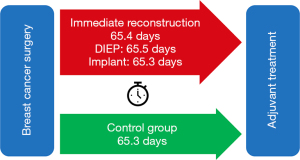
Table 3 shows the time to first adjuvant treatment for either chemotherapy or radiotherapy for the patients in our study cohort.
Table 3
| Reconstruction type | Time to first adjuvant treatment | ||||
|---|---|---|---|---|---|
| Chemotherapy | Radiotherapy | ||||
| Mean (SD) | Median | Mean (SD) | Median | ||
| DIEP | 69.6 (23.5) | 68 | 66.0 (21.1) | 62 | |
| Implant | 56.3 (25.4) | 51 | 75.5 (33.1) | 64 | |
| Total | 62.0 (25.4) | 58 | 70.3 (27.5) | 63 | |
All values in days. SD, standard deviation; DIEP, deep inferior epigastric perforators.
Out of the 168 patients who underwent mastectomy with immediate reconstruction, 20 out of 168 (11.9%) patients had a delay time exceeding 90 days (due to all causes of delay). These patients had a mean time to adjuvant treatment of 117 days, with a maximum delay of 179 days.
We analysed these cases by delay type: 11 out of 20 patients had delays to adjuvant treatment directly related to complications following their immediate breast reconstruction surgery (11 of 168, 6.5%). Out of these 11, 7 patients had implant based immediate breast reconstruction (7 of 168, 4.1%) and 4 patients had autologous reconstruction (4 of 168, 2.4%). As documented in Table 2, 9 out of these 11 patients required a return to theatre for post-operative procedures: 7 patients had implant-based reconstructions and 2 patients were autologous flaps. The remaining 2 of 11 patients (with autologous reconstructions) had minor surgical site related complications that were conservatively managed.
Nine out of the 20 patients that experienced delays, were delayed due to other causes not related to post-surgical complications, as documented in Table 3. In 4 cases it was patients’ choice for personal reasons, including seeking a second opinion, delaying the procedure until after a major festive period, and severe procedure-related anxiety. 1 patient received emergency treatment for a cardiac problem that was unrelated to their surgery and a further 4 patients needed a second stage completion axillary nodal clearance. These latter 4 patients do not qualify as delays, as their completion axillary surgery was necessary prior to deciding on and commencing adjuvant therapy.
Therefore, in our study, there was total of 11 (out of 20) their delays were directly attributed to immediate breast reconstruction surgery (6.5% of the total study population: implant reconstruction 4.1%; autologous reconstruction 2.4%) and a further 9 delays that were unrelated to the immediate breast reconstruction surgery (5.4% of the total study population). In those who had delay due to their reconstructive surgery, mean BMI was 33.8 kg/m2 and 27.2% were smokers.
Discussion
There has been concern that post-operative complications associated with immediate reconstruction may lead to significant and unacceptable delay in delivering adjuvant treatment. In one study, almost 39% of medical oncologists and 23% of surgical oncologists believed that immediate reconstruction can interfere with adjuvant treatments (12). Furthermore, a history of previous radiation therapy was associated with a higher rate of complications in immediate implant reconstruction, which could lead to significant delay in systemic treatment (13). The optimum duration between surgery and initiation of adjuvant therapy has not yet been clearly defined. There are wide ranging recommendations between 31 and 121 days (NICE vs. American Society of Clinical Oncology) to time of delivering adjuvant treatment. Furthermore, there is no clear consensus on whether undertaking immediate reconstruction delays adjuvant therapy. The evidence is contradictory: some prospective studies have found that there was no significant increase in time to adjuvant therapy after immediate reconstruction (14-18). Whereas others have reported delay (19). The effect of ‘perceived’ delay to adjuvant treatment has also been studied with contradictory results. Although some studies have reported that a delay may be associated with worse clinical outcomes (4,20), others found no effect (21).
Our study used the definition for delay to adjuvant treatment as patients receiving their first line of adjuvant treatment more than 90 days after their surgery, as detailed in Table 4. Chavez-MacGregor et al. (20), has shown that time to adjuvant treatment exceeding 90 days is associated with adverse poorer overall survival and patient-recorded outcomes. In our study, the 90-day definition was equivalent to the mean + one standard deviation. In our study however we cannot comment on whether such a delay had any adverse effect on patient outcomes.
Table 4
| Patient | Reconstruction type | Operation year | Time to adjuvant treatment (days) | Cause for delay | Return to theatre |
|---|---|---|---|---|---|
| 1 | DIEP | 2016 | 98 | Skin necrosis, debridement, graft | Yes |
| 2 | DIEP | 2016 | 91 | Skin necrosis, debridement | Yes |
| 3 | DIEP | 2017 | 104 | Superficial infection | No |
| 4 | DIEP | 2018 | 119 | Skin necrosis | No |
| 5 | Implant | 2017 | 144 | Nipple necrosis, washout, revision, implant infection, implant loss | Yes |
| 6 | Implant | 2017 | 97 | Skin necrosis, revision, washout | Yes |
| 7 | Implant | 2018 | 179 | Implant rupture, wound dehiscence, washout | Yes |
| 8 | Implant | 2018 | 121 | Superficial nipple necrosis, washout | Yes |
| 9 | Implant | 2018 | 138 | Skin necrosis, revision, washout | Yes |
| 10 | Implant | 2018 | 142 | Superficial nipple necrosis | Yes |
| 11 | Implant | 2018 | 159 | Dehiscence, skin necrosis | Yes |
PMIBR, post-mastectomy immediate breast reconstruction; DIEP, deep inferior epigastric perforators.
In the control 10.6% experienced delay, corresponding to 9 patients having a time to commencing adjuvant treatment exceeding 90 days, as detailed in Table 5. Comparing the control group to the immediate reconstruction group, it was interesting to observe that a higher number in the control group experienced delay (10.6% compared to 6.5%). Although there was no statistically significant difference between the two groups, we observed that the delay rates due to complications with PMIBR in this single centre study were comparable, if not better, to those in the control group. The impact of observed delay on survival in our study groups is beyond the scope of this paper, but we aim to explore this in future study. Our results support the current practice of offering immediate reconstruction in patients with planned adjuvant therapy.
Table 5
| Patient | Reconstruction type | Operation year | Time to adjuvant treatment (days) | Cause for delay | Return to theatre |
|---|---|---|---|---|---|
| 1 | DIEP | 2016 | 97 | Patient decision: wanted second opinion in alternative medicine | No |
| 2 | DIEP | 2017 | 121 | ANC, declined adjuvant treatment initially | No |
| 3 | DIEP | 2017 | 99 | ANC | No |
| 4 | Implant | 2016 | 102 | Unrelated cardiac treatment | No |
| 5 | Implant | 2016 | 101 | ANC | No |
| 6 | Implant | 2017 | 99 | ANC | No |
| 7 | Implant | 2017 | 119 | Patient decision: sought out second opinion before proceeding | No |
| 8 | Implant | 2018 | 96 | Patient decision: delayed until after festive period | No |
| 9 | Implant | 2018 | 121 | Patient decision: patient anxious about starting adjuvant treatment | No |
PMIBR, post-mastectomy immediate breast reconstruction; DIEP, deep inferior epigastric perforators; ANC, axillary node clearance.
In our series, the immediate reconstruction and control group show similar times to first adjuvant treatment (65.4 vs. 65.3 days, P=0.988). It is therefore safe to conclude that there is no significant delay to adjuvant treatment for patients undergoing immediate breast reconstruction. Furthermore, in the immediate reconstruction group, there were similar times to first adjuvant treatment: 65.3 days for the implant group vs. 65.5 days for the autologous group (P=0.964). It is interesting to highlight that proportion of patients in PMIBR surgery who experienced delay (6.5%) secondary to post-operative complications was lower compared with the control group (10.6%).
We acknowledge the limitations in our study of the small and retrospective population, and short follow-up which precludes of commenting on any detrimental effect of delay in delivering adjuvant treatment.
Conclusions
In conclusion, this study supports existing evidence and current clinical practice that immediate breast reconstruction in patients with breast cancer does not lead to a significant delay to the administration of first adjuvant treatment for both implant-based and autologous reconstructions. Furthermore, we have demonstrated there is no significant difference between implant reconstruction and autologous flap reconstruction in the time to adjuvant treatment. Finally, we observed a lower complication-related delay rate for immediate breast reconstruction when compared to our control group, which we believe warrants further investigation. Our results support our current clinical practice at GSTT. Larger, prospective and longer follow-up studies are needed to provide more insights into this topic—to define ‘delay’ and demonstrate any compromise to patients with regards to timing to adjuvant treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-37/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-37/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-37/coif). AK received speaker fees from Integra. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study adheres to the guidelines on medical protocols and ethics stated in the Declaration of Helsinki (as revised in 2013). The authors assert that ethical approval for publication of this manuscript was not required by their local Ethics Committee. Confidential patient information was rendered anonymous without breaching the duty of confidentiality—the database was independently established for clinical research purposes as per Health Research Authority guidelines. Thus, informed consent and Research Ethics Committee guidance was not necessary for this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sledge GW, Mamounas EP, Hortobagyi GN, et al. Past, present, and future challenges in breast cancer treatment. J Clin Oncol 2014;32:1979-86. [Crossref] [PubMed]
- Jones C, Lancaster R. Evolution of Operative Technique for Mastectomy. Surg Clin North Am 2018;98:835-44. [Crossref] [PubMed]
- Jeevan R, Cromwell D, Browne J, et al. National Mastectomy and Breast Reconstruction Audit. NHS Inf Cent 2011. Available online: https://www.rcseng.ac.uk/library-and-publications/rcs-publications/docs/mastectomy-breast-4/
- Yoon AP, Qi J, Brown DL, et al. Outcomes of immediate versus delayed breast reconstruction: Results of a multicenter prospective study. Breast 2018;37:72-9. [Crossref] [PubMed]
- National Institute for Health and Care Excellence. Early and locally advanced breast cancer: diagnosis and treatment | 1-Guidance | Guidance and guidelines | NICE. NICE Guid 2018. Available online: https://www.nice.org.uk/guidance/ng101
- Pirro O, Mestak O, Vindigni V, et al. Comparison of Patient-reported Outcomes after Implant Versus Autologous Tissue Breast Reconstruction Using the BREAST-Q. Plast Reconstr Surg Glob Open 2017;5:e1217. [Crossref] [PubMed]
- Santosa KB, Qi J, Kim HM, et al. Long-term Patient-Reported Outcomes in Postmastectomy Breast Reconstruction. JAMA Surg 2018;153:891-9. [Crossref] [PubMed]
- Gopie JP, Hilhorst MT, Kleijne A, et al. Women's motives to opt for either implant or DIEP-flap breast reconstruction. J Plast Reconstr Aesthet Surg 2011;64:1062-7. [Crossref] [PubMed]
- Zhong T, Hofer SO, McCready DR, et al. A comparison of surgical complications between immediate breast reconstruction and mastectomy: the impact on delivery of chemotherapy--an analysis of 391 procedures. Ann Surg Oncol 2012;19:560-6. [Crossref] [PubMed]
- Recht A, Comen EA, Fine RE, et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. J Clin Oncol 2016;34:4431-42. [Crossref] [PubMed]
- D'Souza N, Darmanin G, Fedorowicz Z. Immediate versus delayed reconstruction following surgery for breast cancer. Cochrane Database Syst Rev 2011;CD008674. [Crossref] [PubMed]
- Wanzel KR, Brown MH, Anastakis DJ, et al. Reconstructive breast surgery: referring physician knowledge and learning needs. Plast Reconstr Surg 2002;110:1441-50; discussion 1451-4. [PubMed]
- Krueger EA, Wilkins EG, Strawderman M, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys 2001;49:713-21. [Crossref] [PubMed]
- Xavier Harmeling J, Kouwenberg CA, Bijlard E, et al. The effect of immediate breast reconstruction on the timing of adjuvant chemotherapy: a systematic review. Breast Cancer Res Treat 2015;153:241-51. [Crossref] [PubMed]
- Dave R, O'Connell R, Rattay T, et al. The iBRA-2 (immediate breast reconstruction and adjuvant therapy audit) study: protocol for a prospective national multicentre cohort study to evaluate the impact of immediate breast reconstruction on the delivery of adjuvant therapy. BMJ Open 2016;6:e012678. [Crossref] [PubMed]
- Allweis TM, Boisvert ME, Otero SE, et al. Immediate reconstruction after mastectomy for breast cancer does not prolong the time to starting adjuvant chemotherapy. Am J Surg 2002;183:218-21. [Crossref] [PubMed]
- Chang RJ, Kirkpatrick K, De Boer RH, et al. Does immediate breast reconstruction compromise the delivery of adjuvant chemotherapy? Breast 2013;22:64-9. [Crossref] [PubMed]
- Hamahata A, Kubo K, Takei H, et al. Impact of immediate breast reconstruction on postoperative adjuvant chemotherapy: a single center study. Breast Cancer 2015;22:287-91. [Crossref] [PubMed]
- Alderman AK, Collins ED, Schott A, et al. The impact of breast reconstruction on the delivery of chemotherapy. Cancer 2010;116:1791-800. [Crossref] [PubMed]
- Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, et al. Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA Oncol 2016;2:322-9. [Crossref] [PubMed]
- Cold S, Düring M, Ewertz M, et al. Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG). Br J Cancer 2005;93:627-32. [Crossref] [PubMed]
Cite this article as: Antram E, Shaari E, Balasubramanian R, in’t Hout B, Kovacs T, Hamed H, Kothari A. Investigating the time to adjuvant treatment following immediate breast reconstruction in breast cancer patients. Ann Breast Surg 2023;7:15.

