
Surgical complications after immediate breast reconstruction vs. mastectomy alone: impact on the time to delivery of adjuvant therapy
Introduction
Each year, nearly 20.000 women (1) are diagnosed with breast cancer in the Nordic countries and in 2016, approximately 4,500 of these women were Danish (2). Mastectomy is the primary surgical treatment for almost 40% of women, despite improvements in treatment (3). The surgical treatment offered depends on the characteristics of the cancer, the size of the tumor, and the preference of the individual woman. Immediate breast reconstruction (IBR) is increasingly being performed (4).
Breast reconstruction can be performed as an immediate or delayed procedure (5). Both methods attempt to reduce the negative psychological effects of a mastectomy and restore the woman’s body image (6,7). While IBR may lessen the psychological stress a woman is facing, there are, however, concerns that IBR may be associated with a higher risk of post-operative complications, due to the complex procedure and longer operating time, when compared to simple mastectomy. Hence the initiation of adjuvant chemotherapy (ACT) may be postponed.
Delayed ACT has been shown to be associated with an increase in recurrence of breast cancer and worsen patient survival (8,9). Previous studies have indicated that the time from surgery to ACT may be prolonged in patients having a mastectomy with IBR compared to those having a simple mastectomy (10-13). However, the findings are inconsistent, and some studies report no significant differences (14-16).
The primary aim of this study is to compare time from surgery to initiation of ACT in women treated either with mastectomy and IBR or simple mastectomy. The secondary aim was to compare the rate of complications between groups and to define if the choice of procedure and associated complications may impact the time to initiation of ACT. We present the following article in accordance with the STROBE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-113/rc).
Methods
Study design, setting and population
In this retrospective study we reviewed the charts of all women undergoing mastectomy from November 2015 to March 2017 at the Department of Plastic Surgery, Odense University Hospital (OUH). Patients were identified by 4 different ICD10 codes for mastectomy. The ICD10 codes were: KHAC20—total mastectomy, KHAC25—radical mastectomy, KHAC10—nipple areola sparring mastectomy and KHAC15—mastectomy with excision of the nipple areola. Only women treated for breast cancer stage I–III were included. Women with stage IV metastatic cancer or women who received part of their treatment in other hospitals were excluded (Figure 1). Prophylactically treated patients were excluded.

Patients were divided into two groups: (I) women undergoing mastectomy with IBR and (II) simple mastectomy.
Planning of surgical procedure
The medical and surgical treatment options were outlined by the multidisciplinary team consisting of a surgeon, an oncologist, a pathologist, and a radiologist. The treatment was then based on a joint decision by the patient and the breast surgeon. If the women opted for mastectomy and IBR, a plastic surgeon was consulted prior to her final decision. All women had a sentinel node procedure prior to mastectomy to determine the status of the regional lymph nodes. Only women with tumor negative sentinel lymph nodes were candidates for mastectomy and IBR. Women with positive sentinel lymph nodes were offered a simple mastectomy due to the resulting radiotherapy. It is standard practice at our hospital not to offer immediate reconstruction to patients undergoing radiation therapy due to the higher risk of complications and risk of unsatisfying cosmetic result. The standard ACT regimes included three treatments with Epirubicin and Cyclophosphamide followed by nine treatments with Paclitaxel.
All IBR patients were reconstructed using mesh, both biologic and synthetic.
Data collection and outcomes
The primary outcome was comparison of time from surgery to initiation of ACT between the two groups. Days to ACT were defined as days between the mastectomy and the first dose of ACT.
The secondary outcomes were number and types of post-operative complications. The medical records were reviewed to identify major complications. A major complication was defined as one or more of the following complications: skin defect, necrosis, hematoma, infection and red breast syndrome. A skin defect was defined as a defect in the surgical wound that required surgical treatment. These were defined as relevant complications if they occurred within 3 months of surgery, and demanded in-hospital stay, intravenous administered medicine and/or surgical intervention. Data related to postoperative wound healing as previous radiation and neoadjuvant chemotherapy and data related to the surgery including implant type, breast specimen weight and previous breast surgery were also registered.
Study data were collected and managed using REDCap electronic data capture tools hosted at Odense University Hospital (Research Electronic Data Capture) (17).
Data management and statistical analysis
Categorical data were analyzed using Pearson chi-square test or Fisher’s exact test depending on whether the count was greater or lesser than five. Continuous scale variables were visually assessed for normality to determine whether parametric or non-parametric analysis should be performed. Normally distributed data were analyzed using independent-samples t-test, while Wilcoxon signed rank test was used for non-normally distributed data. Numerical data are presented as mean (SD) and categorical data are presented as count (%). All reported P values are two-sided, and the results were considered statistically significant when P<0.05.
To analyze time-to-event data, a Kaplan-Meier curve was plotted and a hazard ratio (HR) was calculated using Cox regression for comparison of days to chemotherapy. Age, hypertension and tissue weight were included as a covariate in the regression. Logistic regression was used to assess risk and estimate odds ratio of complications between mastectomy only and mastectomy with IBR.
If a patient was included twice because of bilateral procedure, all analyses were adjusted with clustered standard errors.
Ethics
The study was approved by the Danish Data Protection Agency (Jr 19/10793) and Danish Patient Safety Authority (Jr 3-3013-2131/1), therefore individual consent for this retrospective analysis was not required. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
Patient characteristics and surgical details
We identified 304 eligible patients, of these 29 were excluded (Figure 1). The remaining 275 women had undergone a total of 314 procedures, of which 57 were prophylactic and excluded. We performed 257 therapeutic procedures and 79 of these had ACT: 20 mastectomies with IBR, and 59 simple mastectomies.
The baseline characteristics and demographics are presented in Table 1. When comparing the simple mastectomy and IBR group, we found that the simple mastectomy group was significantly older (mean 59 vs. 46 years, P<0.001) and had larger mastectomy specimen weight (797 vs. 430 g, P=0.0002). We performed 38 breast reconstructions, 37 with permanent implants and one expander to implant.
Table 1
| Characteristics | Mastectomy + ACT (n=59) | Mastectomy + IBR + ACT (n=20) | P value |
|---|---|---|---|
| Age at surgery (years), mean ± SD | 58.7±10.2 | 45.9±10.7 | <0.001 |
| BMI (kg/m2), mean ± SD | 26.1±4.5 | 24.9±5.2 | 0.346 |
| Tobacco use, n (%) | 16 (27%) | 4 (20%) | 0.767 |
| Hypertension, n (%) | 19 (32%) | 4 (20%) | 0.398 |
| Diabetic, n (%) | 5 (8%) | 1 (5%) | 1.000 |
| Comorbidity*, n (%) | 10 (17%) | 3 (15%) | 1.000 |
| Previous radiation to breast, n (%) | 1 (2%) | 2 (10%) | 0.156 |
| Previous surgery to breast, n (%) | |||
| No | 50 (85%) | 16 (80%) | 0.729 |
| Yes (<30 days) | 3 (5%) | 3 (15%) | 0.167 |
| Tissue weight (g), mean ± SD | 797±408 | 430±196 | 0.0002 |
| Implant type, n (%) | |||
| Expander | – | 1 (3%) | – |
| Permanent | – | 37 (97%) | – |
*, comorbidity: other systemic disease like hypothyroidism, bilateral salpingo-oophorectomy etc. ACT, adjuvant chemotherapy; IBR, immediate breast reconstruction; SD, standard deviation; BMI, body mass index.
Time to delivery of ACT
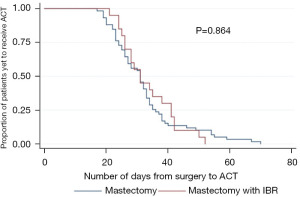
ACT was administered to 79 patients following mastectomy, 59 had a simple mastectomy and 20 mastectomy and IBR, illustrated in Table 2. The time to delivery of ACT was 32 and 33 days for women having simple mastectomy and mastectomy with IBR respectively (P=0.864; Figure 2).
Table 2
| Data | Mastectomy | IBR | P value | HR (95% CI) |
|---|---|---|---|---|
| No. of patients | 59 | 20 | – | – |
| Time to ACT (days), mean (range) | 32 (17 to 70) | 33 (21 to 52) | 0.864 | 1.06 (0.546 to 2.055) |
ACT, adjuvant chemotherapy; IBR, immediate breast reconstruction.
Complication rates
Complication rates for women receiving ACT is shown in Table 3. A higher rate of major complications was found among women receiving IBR and ACT 3/20 (15%), compared to simple mastectomy and ACT 0/59 (0%) (P=0.014; Table 3).
Table 3
| Type of complication | Mastectomy + ACT | IBR + ACT | Odds ratio (95% CI) | Log regression, P value |
|---|---|---|---|---|
| No. of procedures | 59 | 20 | ||
| Total of women with complications, n (%) | 26 (44%) | 9 (45%) | 1.04 (0.37–2.88) | 0.942 |
| Major complication | 0 (0%) | 3 (15%) | NA | 0.014 |
| Skin defect | 4 (7%) | 5 (25%) | 4.58 (1.09–19.22) | 0.037 |
| Necrosis | 7 (12%) | 2 (10%) | 0.83 (0.16–4.34) | 0.821 |
| Hematoma | 12 (20%) | 1 (5%) | 0.21 (0.03–1.70) | 0.142 |
| Infection | 7 (12%) | 4 (20%) | 1.86 (0.48–7.17) | 0.369 |
| Red breast syndrome | 0 (0%) | 1 (5%) | NA | 0.203 |
IBR, immediate breast reconstruction; ACT, adjuvant chemotherapy; NA, not applicable.
The mean follow-up time ranged between 12 and 30 months.
Discussion
We found that women treated with mastectomy and IBR more frequently experienced postoperative complications compared to women treated with simple mastectomy. The complications did not affect the time from surgery to initiation of ACT, when comparing the two groups. Mastectomy followed by IBR is a more demanding and longer procedure than mastectomy alone and as such associated with a higher risk of complications. One would expect that major complications would be associated with a delay in initiation of ACT, which was not the case in our study. This was in line with newly published multicenter study by O’Connell et al. They included 409 women having mastectomy only and 147 women with mastectomy and IBR all receiving ACT. No clinically significant difference in time to delivery of adjuvant therapy was found (3). O’Connell et al. divided their patients into four groups of: (I) mastectomy only, (II) mastectomy and IBR with implant-only technique, (III) mastectomy and IBR with pedicled flaps and (IV) and mastectomy IBR with free-flap technique. They found no significant difference in time to delivery of adjuvant therapy between the groups, although IBR with free-flap technique was associated with longer time to chemotherapy.
One reason and explanation for the lack of prolonged time to ACT despite higher complication rate among the mastectomy + IBR group in this study could be quick diagnosis and treatment of complications by the surgical team to avoid any delay. Similar reflections have been made by other researchers (18). Another possible reason is the small sample size and small overall number of complications in this study.
The complication rates found in this study were comparable with complication rates in previously published studies (10). Sousa et al. (18) had a definition of complications very similar to the definition in this study. In their study group of 315 IBR patients and 401 simple mastectomies, they found a slightly lower complication rate of 13% in the IBR group, and 3% in the mastectomy only group. Zhong et al. (10) looked at a group of 391 patients and found a complication rate of 15.5% for the IBR group and 3.7% for the mastectomy only group. Other studies present either higher or lower complication rates. Chang et al. (16) found that 25.2% of their IBR group (n=107) experienced infection, compared to 15.2% in the mastectomy only group (n=113). Furthermore, 15.9% of the IBR group had to return to surgery for varies reasons, compared to only 1.8% in the mastectomy only group. Hamahata et al. (14) had a very low complication rate of 4% for the IBR group (n=50) and 3% for the mastectomy only group (n=66).
The differences in complication rates among studies might be due to several reasons. (I) Different definitions of complications among the different studies. (II) Variable diligence in registering complications. (III) Different approaches in handling the complications. Some use antibiotics on any suspicion (resulting in a higher registration of infections) while others have a higher threshold, resulting in lower numbers of women registered with treatment of infection. (IV) Different methods of reconstruction—two stage reconstruction with tissue expanders vs. one stage with permanent implants. Many of the above mentioned studies used tissue expanders (10,12,15-19) or did not specify what kind of implants the women were reconstructed with (11,13,14). Sousa et al. (18) used tissue expanders as reference and found that permanent implants have a higher adjusted risk ratio of any complication, though not significant. Of the 38 mastectomies with IBR procedures in this study, only 1 was performed with an expander, the rest were permanent implants. This might explain the relatively small difference in complication rates in this study compared to other studies using expander implants only. It is standard practice at our hospital not to offer IBR to patients treated with radiation therapy, this practice differs from other hospitals and therefor limits the external validity of this study.
Patient selection may explain why no difference in time to delivery of adjuvant therapy was found between our two groups, even though the mastectomy and IBR group experienced a higher incidence of major complications (15% vs. 0%). Our results show that patients receiving mastectomy and IBR were younger, had fewer cases of hypertension and smaller mastectomy specimens. Surgeons are prone to select patients with fewer risk factors for IBR than mastectomy only, in order to minimize complications and prevent any delays in adjuvant therapy (3).
The majority of women with breast cancer is treated with ACT. Questions to how much of a delay in administration, that is acceptable without decreasing survival still exists. A study from 2005 by Cold et al. (20) could not find any survival benefit among Danish breast cancer patients due to early start of ACT within the first 60–90 days after surgery. However, later publications have shown worse outcomes when chemotherapy was delayed for patients with stage III, triple negative or HER2 positive breast cancer (8), and an increased risk of death for patients with a delay over 4 weeks (9). Smith-Graziani et al. conducted a large study documenting that chemotherapy delays are associated with worse survival in older breast cancer patients. They found chemotherapy delays beyond 90 days after surgery negatively affected survival, and that delays were associated with clinical and socioeconomic factors (21).
Despite the fact that nearly all women were reconstructed with permanent implants, resulting in a relatively high rate of complications, we found similar time to delivery of ACT in the two groups. This is in accordance with some of the previous published studies, although most of these include women reconstructed with tissue expanders (3,14-16). The systematic review by Xavier Harmeling et al. (22) and the multicenter study by O’Connell et al. (3) both found that IBR does not delay time to ACT and concludes that IBR is a valid option for breast-cancer patients. However, some studies did find a significant delay among patients receiving IBR (10-13). Eck et al. (15) found similar time to delivery of ACT in the two groups (42 vs. 41 days) but great variations were present. The range of time to delivery of ACT was 8–147 in the mastectomy group and 7–175 in the IBR group. This shows that major complications can severely impact time to ACT. Our corresponding ranges were smaller and in the opposite direction: 17–70 in the mastectomy group vs. 21–52 in the IBR group.
This study has several weaknesses, which must be considered for future studies. Even though, the initial population was large, the sample was small when looking at the specific outcomes. The retrospective design introduces a risk of bias regarding accurate registrations of complications. Furthermore, the higher rate of major complications in the IBR group could be caused by the more tightly controlled follow up scheme compared to the mastectomy group. One of the strengths of this study is the high data completeness. There were very few lacking data, and no recall bias, as data was obtained from medical records recorded at the present time. Patient selection was a problem in some studies where outliners were excluded. However, none of our patients were excluded because of excessively delay to ACT.
Conclusions
We found no difference in time to delivery of ACT when comparing simple mastectomy to mastectomy combined with IBR. Mastectomy combined with IBR using permanent implants seems to be a safe procedure in women with stage I–III breast cancer when treated by an experienced team of breast and plastic surgeons.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-113/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-113/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-113/coif). JBT serves as an unpaid editorial board member of Annals of Breast Surgery from December 2019 to November 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Danish Data Protection Agency (Jr 19/10793) and Danish Patient Safety Authority (Jr 3-3013-2131/1), therefore individual consent for this retrospective analysis was not required. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- NORDCAN. Cancer stat fact sheets. Nordic ccountries. 2017. Available online: http://www-dep.iarc.fr/NORDCAN/english/StatsFact.asp?cancer=200&country=0
- NORDCAN. Cancer stat fact sheets. Denmark - Breast. 2017. Available online: http://www-dep.iarc.fr/NORDCAN/english/StatsFact.asp?cancer=200&country=208
- O'Connell RL, Rattay T, Dave RV, et al. The impact of immediate breast reconstruction on the time to delivery of adjuvant therapy: the iBRA-2 study. Br J Cancer 2019;120:883-95. [Crossref] [PubMed]
- Heidemann LN, Gunnarsson GL, Bille C, et al. Reconstructive breast surgery using implant and mesh. Ugeskr Laeger 2017;179:V10160755. [PubMed]
- Schmauss D, Machens HG, Harder Y. Breast Reconstruction after Mastectomy. Front Surg 2016;2:71. [PubMed]
- Chen W, Lv X, Xu X, et al. Meta-analysis for psychological impact of breast reconstruction in patients with breast cancer. Breast Cancer 2018;25:464-9. [Crossref] [PubMed]
- Zhong T, Hu J, Bagher S, et al. A Comparison of Psychological Response, Body Image, Sexuality, and Quality of Life between Immediate and Delayed Autologous Tissue Breast Reconstruction: A Prospective Long-Term Outcome Study. Plast Reconstr Surg 2016;138:772-80. [Crossref] [PubMed]
- Gagliato DM, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 2014;32:735-44. [Crossref] [PubMed]
- Raphael MJ, Biagi JJ, Kong W, et al. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 2016;160:17-28. [Crossref] [PubMed]
- Zhong T, Hofer SO, McCready DR, et al. A comparison of surgical complications between immediate breast reconstruction and mastectomy: the impact on delivery of chemotherapy--an analysis of 391 procedures. Ann Surg Oncol 2012;19:560-6. [Crossref] [PubMed]
- Vandergrift JL, Niland JC, Theriault RL, et al. Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. J Natl Cancer Inst 2013;105:104-12. [Crossref] [PubMed]
- Lee J, Lee SK, Kim S, et al. Does Immediate Breast Reconstruction after Mastectomy affect the Initiation of Adjuvant Chemotherapy? J Breast Cancer 2011;14:322-7. [Crossref] [PubMed]
- Alderman AK, Collins ED, Schott A, et al. The impact of breast reconstruction on the delivery of chemotherapy. Cancer 2010;116:1791-800. [Crossref] [PubMed]
- Hamahata A, Kubo K, Takei H, et al. Impact of immediate breast reconstruction on postoperative adjuvant chemotherapy: a single center study. Breast Cancer 2015;22:287-91. [Crossref] [PubMed]
- Eck DL, McLaughlin SA, Terkonda SP, et al. Effects of immediate reconstruction on adjuvant chemotherapy in breast cancer patients. Ann Plast Surg 2015;74:S201-3. [Crossref] [PubMed]
- Chang RJ, Kirkpatrick K, De Boer RH, et al. Does immediate breast reconstruction compromise the delivery of adjuvant chemotherapy? Breast 2013;22:64-9. [Crossref] [PubMed]
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. [Crossref] [PubMed]
- Sousa J, Sood R, Liu D, et al. Comparison of Outcomes in Immediate Implant-Based Breast Reconstruction Versus Mastectomy Alone. Plast Surg (Oakv) 2018;26:18-25. [Crossref] [PubMed]
- Munhoz AM, Aldrighi CM, Montag E, et al. Clinical outcomes following nipple-areola-sparing mastectomy with immediate implant-based breast reconstruction: a 12-year experience with an analysis of patient and breast-related factors for complications. Breast Cancer Res Treat 2013;140:545-55. [Crossref] [PubMed]
- Cold S, Düring M, Ewertz M, et al. Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG). Br J Cancer 2005;93:627-32. [Crossref] [PubMed]
- Smith-Graziani D, Lei X, Giordano SH, et al. Delayed initiation of adjuvant chemotherapy in older women with breast cancer. Cancer Med 2020;9:6961-71. [Crossref] [PubMed]
- Xavier Harmeling J, Kouwenberg CA, Bijlard E, et al. The effect of immediate breast reconstruction on the timing of adjuvant chemotherapy: a systematic review. Breast Cancer Res Treat 2015;153:241-51. [Crossref] [PubMed]
Cite this article as: Dissing J, Edvardsen E, Schierbeck J, Thomsen JB, Cold S, Bille C. Surgical complications after immediate breast reconstruction vs. mastectomy alone: impact on the time to delivery of adjuvant therapy. Ann Breast Surg 2023;7:2.

