
Alternative flaps for breast reconstruction: a narrative review on using the thigh, buttocks, and back
Introduction
As the management of breast cancer has dramatically improved in the past decade, so have our techniques for breast reconstruction. Recent innovations in implants and acellular dermal matrices have expanded options for reconstructive surgeons, allowing for cosmetic results previously unattainable in selected cases (1). However, autologous techniques remain to provide unparalleled results in terms of durability and feel for patients (2). Furthermore, recent refinements in technique and ancillary procedures now permit very good matching of a contralateral ptotic breast (3).
Worldwide, the deep inferior epigastric artery perforator (DIEP) flap is the most popular autologous technique used for breast reconstruction. Many patients have abundant tissue at the abdomen, are satisfied with the post-treatment improvement in donor-site contour and find the resulting abdominoplasty-like scar acceptable. However, not every patient is naturally suited for an abdomen-based free flap. With increased anatomical understanding and surgical skills, many other body regions have now become equally good or even better donor regions in selected patients.
Ever since the first studies on autologous breast reconstruction (4), clinicians have written about the reasons as to why some flaps are their first versus the second choice. Determinants include flap-specific donor-site morbidity, expected volume, flap perfusion, technical complexity, flap risk profiles, and suitability for bilateral cases. Some have argued that the medial thigh flaps are the ideal “second” choice whereas others prefer the gluteal region. We believe that such discussions are of limited value since the definition of first and second choice depends on patients’ habitus, preferences, previous procedures, and many other factors, thus varies from patient to patient. At our institution, an academic tertiary referral centre for autologous breast reconstruction, our vision is to provide the most complete array of autologous options to women (5-7). This includes flaps from the inner thigh, lateral thigh, the gluteal and lumbar region in addition to the abdomen (8-11). After thoroughly assessing the preferences, needs, and body type of a patient, pros and cons are discussed of each technique and a joint decision is made regarding which flap, scar locations, and the need for future additional procedures.
In this article, we provide a narrative review on current non-abdomen-based, free flaps for breast reconstruction and share our experience with these flaps. The pros and cons of each flap, patient selection, and key surgical points are highlighted. We present the following article in accordance with the Narrative Review reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-8/rc).
Methods
For this narrative review, we searched PubMed using the following main terms: autologous breast reconstruction, free flap breast reconstruction, alternative flaps for breast reconstruction. We focused on original, English articles that best described surgical technique, perioperative considerations, and outcomes, and used our own experience to complement the findings.
Flaps are grouped by body region and discussed in the following random order: medial thigh, lateral thigh, gluteal region, and lumbar region. Each section provides a short introduction followed by surgical considerations, clinical outcomes, and a future perspective.
Medial thigh I: upper gracilis myocutaneous flaps
Gracilis based myocutaneous flaps are arguably the main flaps that can be harvested from the medial thigh for breast reconstruction (12). Depending on the orientation of the skin island, a transverse upper gracilis (TUG) or a diagonal upper gracilis (DUG) flap can be designed (13). The amount of volume that can be harvested is usually somewhat limited, with reported weights varying between 150–550 grams. In 1992, the musculocutaneous perforators of the TUG were first described and mapped, which led to designing the skin island within the upper third of the gracilis to increase skin viability (14). In the following years, Arnez et al. and Schoeller et al. popularized the technique with their early successful series of TUG flaps for breast reconstruction (15,16). At our institution, we now prefer orienting the skin island diagonally, which allows for a wider skin paddle, less tension on the closure line, lower risk of damaging the lymphatics, and a better-concealed scar. Figure 1 provides a schematic illustration of the flap design and dissection planes.
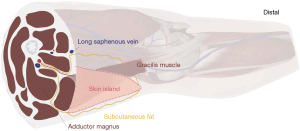
Surgical considerations
Preoperative planning
Upper gracilis myocutaneous flaps are suited for immediate reconstruction after skin-sparing mastectomy cases. The flap may also be used in a delayed or after implant removal.
Gracilis myocutaneous flaps are based on the medial circumflexa femoral vessels, which branch off the profunda femoris. Its pedicle is on average about 8.5 cm inferior to the pubis, located at the anterior border of the gracilis (17). Pedicle length is about 6.7 cm with an average diameter for artery and vein of 2.2, 2.3 mm respectively.
We believe no preoperative angiography is required unless there is a history suggesting potential trauma to the vasculature. In such cases, CT or MR angiography is the preferred imaging modalities with pros and cons.
The medial thigh is marked with the patient standing and externally rotating the thigh to visualize the inguinal and gluteal crease. A transverse skin paddle of up to 30 cm × 10 cm is possible while a diagonal skin paddle can be even designed larger depending on the inner thigh size of the patient and resulting thigh contour (18,19). However one should be aware that in large designs, some flap edges can be poorly perfused and should be discarded. T-shaped skin paddles have also been reported. For the DUG, we mark a line near the intersection of the adductor longus and the thigh perineal crease (most cranial point) towards the medial aspect of the knee, forming the axis of the flap. A symmetrical ellipse is designed around this axis depending on where the largest volume of fat can be recruited and the final estimated scar location. A pinch test is performed to determine the anterior and posterior borders, while ideally ensuring that (I) the anterior border is medial to the femoral neurovascular bundle, (II) the posterior border does not cross the midline of the posterior thigh and (III) the final scar is not visible when standing from the anterior or posterior position. The most distal portion may be discarded if not needed of poorly perfused.
Flap harvest and transfer
Key in safe and efficient flap harvesting is to identify the gracilis muscle early and its pedicle early on. Avoid fat recruitment lateral to the mid axial line to avoid damage to the posterior cutaneous nerve of the thigh. The muscle is typically inset to form the superior pole of the breast. The relatively short pedicle lowers our threshold for partial rib resection for easier anastomosis. We usually anchor the inferior thigh skin flaps to Colles’ fascia to avoid scar problems. Table 1 describes flap harvesting in 10 steps.
Table 1
| Step | Description |
|---|---|
| 1 | Raise flap using an anterior to posterior approach |
| 2 | Dissect through subcutaneous tissue until medial thigh muscles are seen. Usually bevel for more volume. When encountered, preserve the long saphenous vein and preserve femoral triangle lymphatics |
| 3 | Continue flap dissection along anterior border, and identify gracilis muscle |
| Dissect through thigh fascia, along the gracilis muscle anteriorly until circumflexa femoral branch(s) to the gracilis are seen | |
| 4 | Reflect fascia and adductor longus muscle medially to further dissect out gracilis vascular pedicle |
| 5 | Dissect out pedicle towards origin off the profunda femoris. Clip branches to adductor if needed. Further raise flap in suprafascial plane |
| 6 | Raise posterior part of flap, including all the fat above the semitendinosus semimembranosus muscles |
| 7 | Dissect underneath gracilis muscle below the skin island, continue more distally in a suprafascial plane towards the knee until required flap volume is met |
| Clip minor gracilis pedicles if encountered | |
| 8 | To fully raise the flap, transect distal gracilis muscle distally and proximally |
| 9 | If the flap appears venously congested after raising, position the skin island back to its original position and let it rest to prevent undue tension on the pedicle |
| 10 | If ready for transfer, detach the fully elevated flap by clipping the artery and two concomitant veins just distal from their runoff with scissors |
Postoperative key points
Patients recover with knees and hip slightly flexed and head up. Flap monitoring, haemodynamic status, thromboembolic prophylaxis management are performed as with any other free flap breast reconstruction.
Outcomes and future perspective
Upper gracilis myocutaneous free flaps have become an established option for breast reconstruction. These flaps typically have a sufficiently long, predictable pedicle, and are relatively easy to harvest. Its consistency is somewhat similar to that of gluteal free flaps, and it lends itself very well to coning of the flap for more projection (16). Scars, depending on the design of the skin island, can be most times be concealed. They lie somewhat more anterior and superior in comparison with profunda artery-based perforator flaps which are also harvested from the medial thigh area. In comparison, additional volume may be recruited because the gracilis muscle is included.
Flap-specific complications are lymphedema, seroma, wound problems, thigh distortion, bothersome, and aesthetically displeasing scarring. Depending on the definitions used, some groups have reported very high donor-site complication rates up to 62.5% (18-21). We recommend several adjustments we made over the years to reduce such complications (20-22) (Table 1).
One of the largest studies of TUG flaps (n=154 flaps) to date employing the previous modifications reported wound healing rates of only 6% and all temporary sensory deficits at the donor-site in about one-third of cases, underscoring their importance (19). Labial spreading is a very rare but serious complication we have not seen ourselves. Finally, it is worth mentioning that the skin of the medial thigh is relatively darker, which can sometimes contrast the lighter native chest skin in delayed cases.
Ancillary procedures that may broaden indications of the upper gracilis myocutaneous flaps for breast reconstruction are similar to those for other free flap options: lipofilling, adding another free flap, and adding an implant to increase volume. Secondary procedures at a later stage include liposuction, lipofilling, skin and scar refinement, fat necrosis excision, contralateral mastopexy/reduction.
One important gap in knowledge that remains in gracilis based breast reconstructions is flap volume retention over time. It seems reasonable to assume that some muscle atrophy may occur, resulting in loss in original flap volume. Anecdotal evidence and our personal experience suggest this is minimal. However, lack of strong evidence precludes reporting of objective retention outcomes here. Patients should be made aware of this. Nonetheless, gracilis myocutaneous flaps provide natural and pleasing results and we believe that this uncertainty should not preclude women from choosing this option if no other alternatives are available.
Medial thigh II: profunda artery perforator flap
As it is also harvested from the medial thigh, the profunda artery perforator flap is related to upper gracilis based myocutaneous flaps. In comparison with TUG flaps, however, scars lie more posterior and inferior and no muscle is harvested. Moreover, the profunda artery perforator (PAP) flap is a true perforator flap which makes dissection slightly more tedious in our experience. Nevertheless, many women have an unequal fat distribution in the upper medial thigh, on which the decision between a PAP versus gracilis based flap should be based.
The PAP flap builds on previous knowledge on profunda artery-based flaps which had been primarily used for pressure sores and burns (23,24) and uses principles in upper medial thigh lifting. In 2012, Allen et al. expanded on this knowledge and reported on the use of a flap based on the first or second vessels running off the profunda femoris artery that pierced the adductor magnus for breast reconstruction (25). Since then the PAP flap has become a popular flap for patients with small to moderate sized breasts, sufficient posteromedial thigh volume, and an insufficient abdomen.
Although there are typically 3–4 perforating arteries originating from the profunda femoris, the dominant perforator for the PAP is consistently found posterior to the gracilis. The most common location is on approximately 5 cm below the gluteal fold and 3.8 cm from the midline (26). The second most common location is 5 cm below the gluteal fold but about 12 cm from the midline near biceps femoris and vastus lateralis, demonstrating a medial and lateral distribution of the perforators.
Surgical considerations
Preoperative planning
In contrast to gracilis based flaps, we do recommend angiography of the pelvis and lower extremity for PAP flaps to aid in surgical planning. Preoperatively, we note the location at which perforators exit the deep muscular fascia relative to the gluteal fold, the posterior gracilis border and the adjacent muscles. We mark patients standing with an elliptical incision, medially bordered by the adductor longus running laterally along the lateral border of the gluteal fold. Superior border is about 1 cm below the gluteal fold. Inferior border is typically 6, at most 7 cm, below the superior border depending on skin pinch. This is paramount in minimizing wound related problems due to too high tension. Length of the flap varies widely and should also take into account donor-site contour. Figure 2 shows the skin island design.
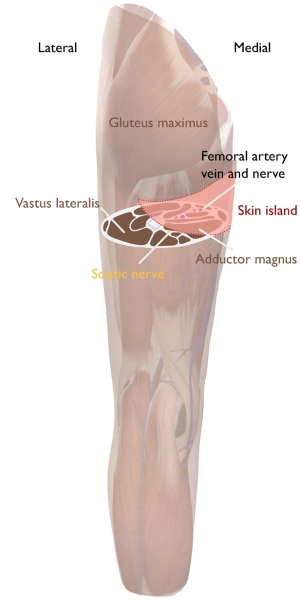
Flap harvest and transfer
The procedure is done in supine position with legs in frog-leg position. Key point in effective PAP flap harvesting is careful preoperative planning using imaging and markings. Also be aware of variations in perforator anatomy, and that the dominant perforator runs caudally within the adductor magnus. Furthermore, dissection is often in a tunnel so control of posterior and side-branches is paramount. If the key adductor magnus perforator is not located, a perforator off the descending branch of the inferior gluteal artery can be used. Table 2 describes each step in PAP flap harvesting in more detail. When raising the flap, we avoid overly aggressive bevelling to avoid postoperative discomfort with sitting on hard surfaces. We routinely use a rib-sparing approach although do not hesitate to resect a rib as needed.
Table 2
| Step | Description |
|---|---|
| 1 | Raise flap using a medial to lateral approach |
| 2 | Incise at medial tip of the flap first, which is near the groin lymphatics and usually overlies the adductor longus muscle |
| 3 | Develop flap towards lateral. Do not bevel superiorly. Bevel inferiorly as needed. Identify gracilis muscle, open fascia in the direction of the fascia fibers at posterolateral portion, and dissection further |
| 4 | Retract gracilis anteriorly, identify and dissect through adductor magnus fascia and muscle |
| 5 | Proceed subfascial dissection posteriolaterally until perforator is found |
| 6 | Continue intramuscular perforator dissection to origin on profunda femoral artery |
| 7 | Reposition retractors regularly, and create sufficient exposure. If loose areolar plane behind adductor muscle is reached, pedicle length may be sufficient and dissection may be sufficient in some cases |
| 8 | Posterior, suprafascial dissection, flap detachment |
| 9 | Perform microsurgical anastomosis |
| 10 | Perform flap shaping or coning, inset, and pocket contouring as needed |
Postoperative key points
Patients are instructed to start sitting on the first postop day and ambulating. Discharge occurs typically at day 4 or 5. Flap monitoring, hemodynamic status, thromboembolic prophylaxis is managed as any other free flap breast reconstruction.
Outcomes and future direction
PAP flaps can deliver great breast reconstruction results in selected patients, with very low failure rates. Compared with gracilis based flaps, it usually has a longer pedicle, and no muscle harvest is required. Projection is easily achieved because the design of the flap allows for great coning. Average flap weights of around 400 grams can be achieved with judicious bevelling, making the flap particularly suited for small to moderate sized breasted patients. Scars are well-hidden and not bothersome particularly if the flap is well designed.
The main disadvantages of the PAP flap relate to the donor site, including wound healing problems, and surgical site infections occurring in 3.6% and 8.2% of cases respectively (27,28). Patients also can report sitting transient discomfort up to 3 months. One of the largest studies to date has reported no lymphedema occurrences following flap harvest. Since it’s the first report of its use, the PAP flap has proved to be a great, reliable, and safe option for autologous breast reconstruction. Nonetheless, we feel that few reconstructive microsurgeons consider this flap routinely. We, therefore, believe great opportunity lies with increasing the awareness about the versatility of this flap both for breast reconstruction and other indications (29).
Gluteal region: superior gluteal artery perforator (SGAP) flap
The first report of using the gluteal region for breast reconstruction was by Fujino et al. in 1975 who used a gluteal myocutaneous flap (30). However, despite large initial interest in this flap, it fell out of favour due to the risk of sciatic nerve injury and technical difficulties associated with flap harvest. It was only after the concept of perforator flaps became well-established that the gluteal artery perforator (GAP) flaps made their re-entry (31-33).
Thin patients seeking autologous reconstruction who accept scars and deformity in the gluteal region are potential candidates. We avoid gluteal artery perforator flaps in the severely obese because the bulk of the flap to pedicle ratio increases. In comparison to other non-abdominal donor sites such as the medial thigh, the gluteal region usually allows for a larger volume harvested. It should be noted that the short pedicle length of the perforator and its size mismatch with recipient vessels can be demanding. Below we describe the SGAP flap. Despite having a shorter pedicle than the inferior gluteal artery perforator (IGAP) flap, we prefer the SGAP because it allows for the entire procedure to be done in supine position in selected cases using the modifications previously published by our group (5).
Surgical considerations
Preoperative planning
Although gluteal perforators are very consistent, we routinely perform contrast angiography for efficiency purposes. Markings are done with the patient in standing and/or prone position using a doppler device. Axis of the flap is slightly oblique or more horizontally oriented. Flap width is usually between 8–12 cm. We mark an elliptical incision, with the medial border usually slightly higher than the mid-gluteal crease. Figure 3 shows the skin island design.
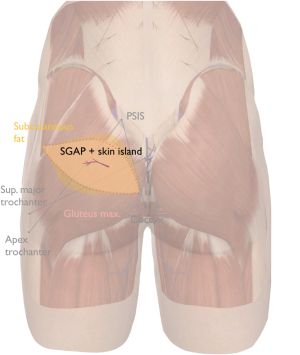
The superior gluteal artery (SGA) is the largest branch of the internal iliac artery. It exits the pelvis through sciatic foramen above piriformis and inferior to gluteus medius. This exit point is at the junction of the proximal and middle thirds connecting the posterior superior iliac spine (PSIS) to the apex of the greater trochanter. Alternatively, this point is about 6 cm from PSIS, and 4.5 cm lateral to the mid-sacrum. Once the SGA leaves the pelvis, it divides into a superficial and deep branch. The superficial branches that enter below and perforate the gluteus maximus muscle towards the skin are typically dissected in the classical SGA perforator flap. However, if present, we select the septocutaneous perforators that run with the gluteus medius fascia at the superolateral edge of the gluteus maximus (5). This allows harvesting the flap completely in supine position. On average, SGAPs have a pedicle length of 9.8 cm, run intramuscularly for 5.3 cm and a diameter ranging from 0.9–1.5 mm, which is small in comparison with the medial thigh flaps (34).
Flap harvest and transfer
Key point in effective SGAP flap harvesting is centering the flap over identified perforators, initial suprafascial and later subfascial dissection after the periperforator area is encountered, and meticulous dissection when encountering the subgluteal fat plane for additional length and prevention of deep bleeding. Patients are typically positioned in lateral decubitus for unilateral cases and while a supine-prone-supine sequence is required in bilateral reconstruction. Table 3 describes the steps in more detail. However, as previously described, the procedure can be done completely in supine position in selected cases.
Table 3
| Step | Description |
|---|---|
| 1 | Make incision first superiorly, inferiorly, and laterally. If desired, identify cluneal nerves at superior border and include them for sensate reconstruction |
| 2 | Typically, bevel away from flap marking for better contour of the flap and additional volume recruitment |
| 3 | Raise flap from a lateral to medial fashion, suprafascially |
| 4 | Dissect more medially above gluteus maximus until area of superior gluteal artery perforators is reached |
| 5 | Incise fascia in the direction of the fibers at this point for subfascial dissection for better visualisation of perforators [1–3] |
| 6 | Reposition retractors regularly, and create sufficient exposure |
| Split gluteus muscle in the muscle fiber direction as much as possible | |
| 7 | Open posterior fascia of the gluteus maximum, expose subgluteal fatpad, place retractors in gluteus medius and/or piriformis muscles when encountered for more exposure |
| 8 | Slow down in pedicle dissection, and carefully manage often encountered combinations of intricate small and larger vascular branches |
| 9 | If needed, maximize pedicle length but consider that (I) dissection in the subgluteal fat can only provide 2–3 cm additional length, and that (II) the deepest part of the pedicle lies along periosteum of the pelvis which makes it susceptible to difficult bleeding |
| 10 | Once the perforator is fully dissected towards the superior gluteal artery, the remainder of the medial incision can be completed, and the flap is isolated |
| In closure of the donor site, avoid undermining over greater trochanter and iliac crest |
Postoperative key points
Patients are instructed to start ambulating on postop day 2. Discharge occurs typically at day 4 or 5. Flap monitoring, hemodynamic status, thromboembolic prophylaxis is done as any other free flap breast reconstruction.
Outcomes and future perspective
When used judiciously, SGAP flaps provide aesthetically very pleasing results (35). Gluteal fat is typically firmer than natural breast parenchyma due to a developed reticular system. This allows for great projection but shaping can be more difficult as the tissue is less pliable.
Despite advances in our anatomical understanding and surgical skills, gluteal perforator flaps remain one of the most challenging free flaps with reported flap failure rates up to 8% even in the most experienced hands (33,35). Wound problems may be seen up to 6% of cases, and seroma in as many as 13.5%. A recent observational study found that patients undergoing SGAP reconstruction were less satisfied than those receiving a DIEP flap, concluding that we may have underestimated the donor site morbidity of the SGAP flap (36). The authors reported that the lumbar flap has therefore replaced the SGAP in their practice, while we still routinely perform both in our own. Depending on patient preference and their condition, we do perform one-stage bilateral SGAP reconstructions in suitable cases.
One direction for future studies is to compare the classical method of harvesting SGAP with raising the flap on its septocutaneous perforators, which prevents cumbersome and risky positional changes during surgery. These studies should focus on procedural outcomes such as operative time and donor site related outcomes as we feel that this modification allows more favourable placement the scar.
Lateral thigh: the lateral thigh perforator (LTP) flap
The lateral thigh was introduced as a donor site region for breast reconstruction in 1990 with the musculocutaneous tensor fascia lata free flap (37). In the following years, a variation of this flap without muscle or fascia was popularized as the LTP flap for breast reconstruction (38). Increased understanding regarding perforator anatomy has led us to favour selecting the septocutaneous vessels that run in the posterior septum when possible (5,39). We routinely consider the LTP flap in women with minimal abdominal but abundant upper lateral thigh volume who can accept a scar in this region.
Surgical considerations
Preoperative planning
The lateral femoral circumflex artery forms the basis of all variants of the LTP flap. Its septocutaneous perforators are more constant and larger than the musculocutaneous perforators. Average length of the septocutaneous perforators is about 7–8 cm (40). Although the septocutaneous perforators located in the posterior septum between the TFL and gluteus medius muscles are predictable and relatively easy to dissect, the pedicle may be relatively short with typically a small artery and friable vein. Figure 4 and Figure 5 show the skin island design and perforator identification, respectively.
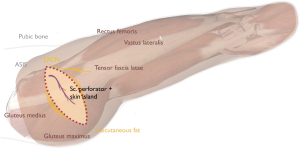
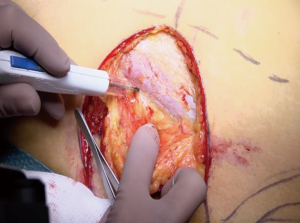
Flap harvest and transfer
Key point in effective LTP flap harvesting are accurate preoperative markings. Anterior border of the flap is determined by a line that runs from anterior superior iliac spine (ASIS) to the superolateral patella. Then a horizontal line is marked parallel to the top edge of the symphysis pubis which the height at which most perforators can be found. Further estimation of exact perforator location is based on angiography measurements of distances between the perforators and the ASIS and single endplates penetrating screw (SEPS). Once located, we then design a horizontal elliptical flap which may be designed with either an upward or downward slant towards posterior depending on the fat distribution. Pinch test is used to confirm the markings. Table 4 details each step.
Table 4
| Step | Description |
|---|---|
| 1 | Raise flaps from anterior tot posterior, dissecting suprafascially over the TFL |
| 2 | Preserve lateral femoral cutaneous nerve when encountered, and other cutaneous nerves if possible |
| 3 | Continue dissection until the TFL-gluteus medius posterior septum is encountered |
| 4 | Divide musculocutaneous perforators of the TFL unless angiography suggests otherwise |
| 5 | Identify (septocutaneous) perforator(s) of interest |
| 6 | Discontinue further posterior dissection to prevent tension |
| 7 | Access posterior septum by incising the TFL fascia |
| 8 | Retract TFL, rectus femoris, and Sartorius muscles for sufficient exposure of pedicel dissection |
| 9 | Dissect pedicle towards origin of LCFA, although sufficient length is typically achieved before this is reached |
| 10 | Close donor site using progressive tension quilting sutures and limited undermining |
TFL, tensor fascia lata; LCFA, lateral circumflex femoral artery.
Postoperative key points
Patients may mobilise on postop day 2–3. Flap monitoring and drains are managed as standard.
Outcomes and future perspective
LTP flaps can be used to achieve very pleasing results, are reliable and offer low failure rates in experienced hands (41). The lateral thigh fat is somewhat firmer than abdominal fat, yet more supple compared to gluteal fat. This firmness allows for good projection to be achieved. With proper patient counselling and selection, the majority of patients find the postoperative scars at the lateral upper thigh very acceptable. Another advantage is, as compared with lumbar or gluteal flaps, no positional change is required, and no interposition grafts are required in spite of the relatively short pedicle. Lastly, we also consider the relative ease of dissection of the septocutaneous perforators a major advantage.
Although we have seen transient numbness in the lateral thigh region, this can be avoided by preserving the lateral femoral cutaneous nerve in most cases. In our experience donor site complaints are fewer and less severe than those of medially based thigh flaps such as the PAP or TUG or gluteal flaps. Secondary corrections such as liposuction or fat grafting both at the breast and donor site are often required to optimize contour and volume. If one LTP flap produces too little volume, two flaps may be stacked to achieve satisfactory volume (42). Bilateral cases can be done in a single stage with relative ease.
As present, we feel that the LTP is somewhat under-recognized as an attractive autologous option for breast reconstruction. Future comparative studies are needed to test the abovementioned advantages in comparison with other non-abdomen-based flaps. We feel that such evidence is needed to increase awareness for this flap, which ultimately may translate into more options for those who lack a suitable abdominal donor site.
Lumbar region: the lumbar artery perforator flap
Before the first report on a lumbar artery perforator flap for breast reconstruction by de Weerd et al. in 2003, flaps from the lumbar region were mostly used as pedicled musculocutaneous or fasciocutaneous flaps to treat pressure sores or other defects in this region (43). From early studies on these pedicled flaps, its short pedicle length of about 4 cm became known (44). Since 2003, the free LAP flap has gradually gained popularity, mostly in expert microsurgical centers (45).
Thin patients who have insufficient abdominal tissue and can accept a scar in the lumbar region are potential candidates. Scars usually lie outside the underwear area and, when appropriately placed, allow for aesthetic contouring the flank. In unilateral cases, liposuction is often needed to symmetrize the flanks. Lumbar fat is usually firmer than that from the abdomen but more pliable and softer than gluteal fat. We believe that this resembles the feel of breast parenchyma very well. While the possibility for flank contouring and intrinsic tissue feel are clear advantages of the LAP flap, these need to be balanced against the advanced surgical skills required for this flap due to the short pedicle, small perforator diameter, and the need for a vascular interposition graft.
Surgical considerations
Preoperative planning
We perform contrast angiography routinely to assess the position and configuration of the lumbar artery perforators. For bilateral cases, we typically stage the reconstruction with a minimum of 3 months between each side.
Markings are done with the patient in standing position using a doppler device. Figure 6 shows flap orientation. Perforators are sought and confirmed with doppler with the midline and iliac crest as landmarks. Axis of the flap is slightly oblique oriented. Dominant skin perforators originate from lumbar arteries at the 3rd or 4th vertebra. The maximum skin resection is determined per pinch testing. A gluteal extension is considered for extra volume recruitment. We follow a supine-prone-supine positional sequence.
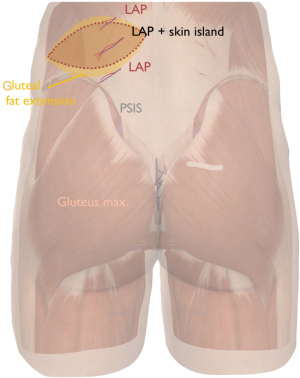
Flap harvest and transfer
Key point in harvesting LAP flaps include orienting the skin markings based on key landmarks and preparing the surgical team members to ensure efficient, twice repositioning of the patient. We routinely perform the procedure with two teams. Table 5 describes each step in more detail. As mentioned, the pedicle is typically very short and has a size mismatch with the acceptor vessels. As such, we recommend using a composite vascular interposition graft such the deep inferior epigastric artery and vein if still available. In tertiary cases, the surgeon can often times harvest the graft through an existing abdominal scar from a previously failed reconstruction.
Table 5
| Step | Description |
|---|---|
| 1 | Prepare the operative team for a supine-prone-supine operative sequence |
| 2 | Consider a two team approach: the primary surgeon harvesting the composite interposition graft (usually deep inferior epigastric artery and vein harvested as standard), the secondary surgeon starting at the thorax |
| 3 | Typically, we plan for ipsilateral harvest. Check skin resection using pinch test, raise flaps from medial to lateral with surgeon standing on the opposite side |
| 4 | Bevel caudally to include more gluteal fat for better contour and more upper pole fullness if needed |
| 5 | Identify lumbar artery perforators which usually arise from the interval between erector spinae muscles and quadratus lumborum. Note: these perforators are more tightly encased by the fascias so identification is more difficult than other free flaps |
| 6 | Once perforator(s) are identified, open surrounding fascias, and complete perforator dissection between or through the muscles. If the pedicle is small or friable, we will harvest it along with a cuff of fascia. As an interpositional graft is typically used, do not pursue maximum perforator length to prevent nerve root injury and difficult deep dissection at the transverse process |
| 7 | Perform anastomosis (primary team) between the cranial, smaller caliber end of the interpositional graft to the lumbar artery perforator on a separate table |
| 8 | During donor site closure, the other team performs multilayer closure of donor site using quilting sutures, drains, and a vest over pants technique as indicated to prevent high risk of seroma |
| 9 | After repositioning the patient to supine position, the second anastomosis between the caudal, larger caliber end of the interpositional graft and the recipient vessels are done as standard |
| 10 | Further flap inset and shaping is done, which is only required in a limited amount of cases |
Outcomes and future direction
LAP flaps can mimic breast greatly because lumbar fat provides an ideal combination of pliability, projection, and firmness that resembles breast parenchyma. However, as mentioned above, its relatively short pedicle and vessel calibre mismatch make it technically a very demanding flap. Opsomer et al. have reported a revision rate of 22% and a 9% flap failure rate using this approach in one of the largest series to date (45). Although acceptable, these numbers mandate that we believe a lumbar flap should not be used if the abdomen is suited as a donor site. At our centre, we have used the LAP flap primarily in tertiary cases.
Although great results may be achieved with LAP flaps, surgeons should be aware of several flap-specific outcomes and donor site problems that can occur. Flank seroma is notorious after LAP flaps, which may be minimized through quilting sutures. Furthermore, donor-site pain seems to occur more frequently than with other free flaps, which we aim to minimize through limited undermining of the flanks for closure and timely discontinuation of pedicle dissection once the transverse process of the vertebra is reached. A recent comparative study has reported lower absolute BREAST-Q subdomain scores for donor site well-being and donor site appearance after lumbar flaps as compared with DIEP and SGAP donor sites, suggesting that patients may weigh more heavily on such donor site problems than previously thought (36). With proper patient selection and design, on average, the number of secondary corrections is similar to other flaps such as the DIEP (46). Moreover, LAP flaps can be made sensate by including the superior cluneate nerves. Lastly, although one-stage bilateral breast reconstruction with LAP flaps can be done, the risks of a very long procedure need to be weighed against the disadvantage of having to undergo a second procedure. We recommended to stage bilateral cases with at least 3 months in between the two procedures rather than doing two LAP flaps in one stage. However, with expected improvements in microsurgical techniques in the future, we hope one day to achieve similarly flap success rates with these flaps as with the previously mentioned other free flaps.
General discussion
To date, many surgeons counsel women who seek breast reconstruction yet lack sufficient abdominal tissue for a DIEP flap towards implant-based reconstructions. This article aimed to share a current perspective on flaps that can be harvested from body regions other than the abdomen, such as the upper thigh, gluteal and lumbar regions. For those interested in learning to perform any of these flaps, a 10-step summary is provided describing our technique in flap harvesting in more detail. Furthermore, key findings of influential studies were discussed to highlight the flap-specific advantages and caveats.
With appropriate patient selection, preoperative preparations, and surgical technique all flaps discussed in this article may be used to reconstruct an aesthetically pleasing breast mound (2). Nonetheless, each technique has unique trade-offs. For example, gracilis based myocutaneous flaps are harvested with relative ease and allow for medial thigh contouring (13). However, the extent to which these flaps lose volume over time remains uncertain. In comparison, gluteal artery perforator flaps typically provide greater volume and easier projection but are technically more demanding due to shorter and smaller sized pedicle (35). For some patients, the lumbar artery perforator flap may be the right or even only choice, which allows for a completely hidden scar from a frontal view among other advantages (43,45). However, patients should be made aware that this flap confers the highest flap failure rate because it is technically very demanding even for the most adept microsurgeons. As such, we believe that lumbar flaps are most suited for tertiary cases. The surgeon specializing in autologous reconstruction needs to understand all these flaps and techniques in order to counsel patients to the one best suited to their preferences, habitus, and goals. That said, we acknowledge that it requires years of focused practice to become adept at all of them. We therefore advocate the philosophy where patients should be referred either internally or extramurally to the colleague known to be most skilled at performing a specific technique.
A limitation of the current narrative review is selection bias; it is likely that personal preference has led to inclusion of certain flaps other experienced surgeons would have chosen differently. For example, we are aware that some surgeons favour the IGAP flap over the SGAP because it has a slightly longer pedicle or rarely use the LTP of its impact on the donor site (31). However, this review was intended from the outset as a discussion of different techniques incorporating personal perspectives that were gained through years of microsurgical experience. Nonetheless, to facilitate an objective discussion of the outcomes of each technique, we included key findings from current literature. A second limitation is the lack of randomized trials comparing two or more reconstructive techniques. For example, one may question the validity of comparing the much higher flap failure rate of the lumbar artery perforator flap if these are primarily done in tertiary cases whereas studies examining the other flaps were done in less complex cases. Randomisation would have eliminated this confounding by indication bias, resulting in a more valid comparison. Nonetheless, we are all aware of the practical and ethical issues associated with randomisation at an individual patient level. This is particularly the case in breast reconstruction science as no single patient is exactly the same concerning her preferences and habitus. Future investigators might consider adopting a clustered randomised trial design instead, in which the unit of randomisation is at an institutional level rather than the individual patient (i.e., surgeons or practices that focus on DIEP flaps versus those who perform other flaps) (47,48).
Conclusions
In the past decades, few subfields in plastic surgery have seen the advancements as large as in autologous breast reconstruction. New flaps have been pioneered while old techniques have been refined to the point where we now strive for aesthetic outcomes that parallel those of cosmetic breast and body contouring surgery. As shown in this article, one can harvest regions other than the abdomen to reconstruct natural breasts with acceptable risk, including the thigh, buttocks and back. We are confident that many advancements in design and surgical technique remain to follow, which makes autologous breast reconstruction a hugely exciting field. It is our sincere hope that articles such as the present one will (I) inspire both patients and surgeons to look beyond the abdomen when discussing breast reconstruction and (II) accelerate the learning process for the future generation of reconstructive surgeons in performing these flaps.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard and Jørn Bo Thomsen) for the series “Breast Reconstruction—The True Multidisciplinary Approach” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-8/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-8/coif). The series “Breast Reconstruction—The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DeLong MR, Tandon VJ, Farajzadeh M, et al. Systematic Review of the Impact of Acellular Dermal Matrix on Aesthetics and Patient Satisfaction in Tissue Expander-to-Implant Breast Reconstructions. Plast Reconstr Surg 2019;144:967e-74e. [Crossref] [PubMed]
- Chang EI. Latest Advancements in Autologous Breast Reconstruction. Plast Reconstr Surg 2021;147:111e-22e. [Crossref] [PubMed]
- Patel NG, Rozen WM, Chow WT, et al. Stacked and bipedicled abdominal free flaps for breast reconstruction: considerations for shaping. Gland Surg 2016;5:115-21. [PubMed]
- Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg 1979;13:423-7. [Crossref] [PubMed]
- Tuinder S, Chen CM, Massey MF, et al. Introducing the septocutaneous gluteal artery perforator flap: a simplified approach to microsurgical breast reconstruction. Plast Reconstr Surg 2011;127:489-95. [Crossref] [PubMed]
- Beugels J, Vasile JV, Tuinder SMH, et al. The Stacked Hemiabdominal Extended Perforator Flap for Autologous Breast Reconstruction. Plast Reconstr Surg 2018;142:1424-34. [Crossref] [PubMed]
- Beugels J, Cornelissen AJM, van Kuijk SMJ, et al. Sensory Recovery of the Breast following Innervated and Noninnervated DIEP Flap Breast Reconstruction. Plast Reconstr Surg 2019;144:178e-88e. [Crossref] [PubMed]
- Bordianu A, Leoveanu I, Chang EI. Autologous breast reconstruction beyond the DIEP: a narrative review of autologous breast reconstruction options beyond the DIEP flap. Ann Breast Surg 2020;4:15. [Crossref]
- Healy C, Allen RJ Sr. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J Reconstr Microsurg 2014;30:121-5. [PubMed]
- Hunter C, Moody L, Luan A, et al. Superior Gluteal Artery Perforator Flap: The Beauty of the Buttock. Ann Plast Surg 2016;76:S191-5. [Crossref] [PubMed]
- Hamdi M, Craggs B, Brussaard C, et al. Lumbar Artery Perforator Flap: An Anatomical Study Using Multidetector Computed Tomographic Scan and Surgical Pearls for Breast Reconstruction. Plast Reconstr Surg 2016;138:343-52. [Crossref] [PubMed]
- Vega SJ, Sandeen SN, Bossert RP, et al. Gracilis myocutaneous free flap in autologous breast reconstruction. Plast Reconstr Surg 2009;124:1400-9. [Crossref] [PubMed]
- Park JE, Alkureishi LWT, Song DH. TUGs into VUGs and Friendly BUGs: Transforming the Gracilis Territory into the Best Secondary Breast Reconstructive Option. Plast Reconstr Surg 2015;136:447-54. [Crossref] [PubMed]
- Yousif NJ, Matloub HS, Kolachalam R, et al. The transverse gracilis musculocutaneous flap. Ann Plast Surg 1992;29:482-90. [Crossref] [PubMed]
- Arnez ZM, Pogorelec D, Planinsek F, et al. Breast reconstruction by the free transverse gracilis (TUG) flap. Br J Plast Surg 2004;57:20-6. [Crossref] [PubMed]
- Schoeller T, Wechselberger G. Breast reconstruction by the free transverse gracilis (TUG) flap. Br J Plast Surg 2004;57:481-2. [Crossref] [PubMed]
- Wong C, Mojallal A, Bailey SH, et al. The extended transverse musculocutaneous gracilis flap: vascular anatomy and clinical implications. Ann Plast Surg 2011;67:170-7. [Crossref] [PubMed]
- Locke MB, Zhong T, Mureau MA, et al. Tug 'O' war: challenges of transverse upper gracilis (TUG) myocutaneous free flap breast reconstruction. J Plast Reconstr Aesthet Surg 2012;65:1041-50. [Crossref] [PubMed]
- Schoeller T, Huemer GM, Wechselberger G. The transverse musculocutaneous gracilis flap for breast reconstruction: guidelines for flap and patient selection. Plast Reconstr Surg 2008;122:29-38. [Crossref] [PubMed]
- Buchel EW, Dalke KR, Hayakawa TE. The transverse upper gracilis flap: Efficiencies and design tips. Can J Plast Surg 2013;21:162-6. [Crossref] [PubMed]
- Saint-Cyr M, Wong C, Oni G, et al. Modifications to extend the transverse upper gracilis flap in breast reconstruction: clinical series and results. Plast Reconstr Surg 2012;129:24e-36e. [Crossref] [PubMed]
- Wechselberger G, Pülzl P, Schoeller T. Re: The transverse myocutaneous gracilis flap: Technical refinements. J Plast Reconstr Aesthet Surg 2010;63:e711-2. [Crossref] [PubMed]
- Song YG, Chen GZ, Song YL. The free thigh flap: a new free flap concept based on the septocutaneous artery. Br J Plast Surg 1984;37:149-59. [Crossref] [PubMed]
- Angrigiani C, Grilli D, Siebert J, et al. A new musculocutaneous island flap from the distal thigh for recurrent ischial and perineal pressure sores. Plast Reconstr Surg 1995;96:935-40. [Crossref] [PubMed]
- Allen RJ, Haddock NT, Ahn CY, et al. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg 2012;129:16e-23e. [Crossref] [PubMed]
- Haddock NT, Greaney P, Otterburn D, et al. Predicting perforator location on preoperative imaging for the profunda artery perforator flap. Microsurgery 2012;32:507-11. [Crossref] [PubMed]
- Allen RJ Jr, Lee ZH, Mayo JL, et al. The Profunda Artery Perforator Flap Experience for Breast Reconstruction. Plast Reconstr Surg 2016;138:968-75. [Crossref] [PubMed]
- Haddock NT, Gassman A, Cho MJ, et al. 101 Consecutive Profunda Artery Perforator Flaps in Breast Reconstruction: Lessons Learned with Our Early Experience. Plast Reconstr Surg 2017;140:229-39. [Crossref] [PubMed]
- Largo RD, Chu CK, Chang EI, et al. Perforator Mapping of the Profunda Artery Perforator Flap: Anatomy and Clinical Experience. Plast Reconstr Surg 2020;146:1135-45. [Crossref] [PubMed]
- Fujino T, Harasina T, Aoyagi F. Reconstruction for aplasia of the breast and pectoral region by microvascular transfer of a free flap from the buttock. Plast Reconstr Surg 1975;56:178-81. [Crossref] [PubMed]
- Boustred AM, Nahai F. Inferior gluteal free flap breast reconstruction. Clin Plast Surg 1998;25:275-82. [Crossref] [PubMed]
- Allen RJ, Levine JL, Granzow JW. The in-the-crease inferior gluteal artery perforator flap for breast reconstruction. Plast Reconstr Surg 2006;118:333-9. [Crossref] [PubMed]
- Guerra AB, Metzinger SE, Bidros RS, et al. Breast reconstruction with gluteal artery perforator (GAP) flaps: a critical analysis of 142 cases. Ann Plast Surg 2004;52:118-25. [Crossref] [PubMed]
- Georgantopoulou A, Papadodima S, Vlachodimitropoulos D, et al. The microvascular anatomy of superior and inferior gluteal artery perforator (SGAP and IGAP) flaps: a fresh cadaveric study and clinical implications. Aesthetic Plast Surg 2014;38:1156-63. [Crossref] [PubMed]
- Baumeister S, Werdin F, Peek A. The sGAP flap: rare exception or second choice in autologous breast reconstruction? J Reconstr Microsurg 2010;26:251-8. [Crossref] [PubMed]
- Opsomer D, Vyncke T, Ryx M, et al. Comparing the Lumbar and SGAP Flaps to the DIEP Flap Using the BREAST-Q. Plast Reconstr Surg 2020;146:276e-82e. [Crossref] [PubMed]
- Elliott LF, Beegle PH, Hartrampf CR Jr. The lateral transverse thigh free flap: an alternative for autogenous-tissue breast reconstruction. Plast Reconstr Surg 1990;85:169-78; discussion 179-81. [Crossref] [PubMed]
- Kind GM, Foster RD. Breast reconstruction using the lateral femoral circumflex artery perforator flap. J Reconstr Microsurg 2011;27:427-32. [Crossref] [PubMed]
- Tuinder S, Baetens T, De Haan MW, et al. Septocutaneous tensor fasciae latae perforator flap for breast reconstruction: radiological considerations and clinical cases. J Plast Reconstr Aesthet Surg 2014;67:1248-56. [Crossref] [PubMed]
- Maricevich MA, Bykowski MR, Schusterman MA 2nd, et al. Lateral thigh perforator flap for breast reconstruction: Computed tomographic angiography analysis and clinical series. J Plast Reconstr Aesthet Surg 2017;70:577-84. [Crossref] [PubMed]
- Tuinder SMH, Beugels J, Lataster A, et al. The Lateral Thigh Perforator Flap for Autologous Breast Reconstruction: A Prospective Analysis of 138 Flaps. Plast Reconstr Surg 2018;141:257-68. [Crossref] [PubMed]
- Tessler O, Guste J, Bartow MJ, et al. Stacked Lateral Thigh Perforator Flap as a Novel Option for Autologous Breast Reconstruction. Plast Reconstr Surg 2019;143:1601-4. [Crossref] [PubMed]
- de Weerd L, Elvenes OP, Strandenes E, et al. Autologous breast reconstruction with a free lumbar artery perforator flap. Br J Plast Surg 2003;56:180-3. [Crossref] [PubMed]
- Roche NA, Van Landuyt K, Blondeel PN, et al. The use of pedicled perforator flaps for reconstruction of lumbosacral defects. Ann Plast Surg 2000;45:7-14. [Crossref] [PubMed]
- Opsomer D, Stillaert F, Blondeel P, et al. The Lumbar Artery Perforator Flap in Autologous Breast Reconstruction: Initial Experience with 100 Cases. Plast Reconstr Surg 2018;142:1e-8e. [Crossref] [PubMed]
- Opsomer D, Vyncke T, Depypere B, et al. Lumbar Flap versus the Gold Standard: Comparison to the DIEP Flap. Plast Reconstr Surg 2020;145:706e-14e. [Crossref] [PubMed]
- Hemming K, Eldridge S, Forbes G, et al. How to design efficient cluster randomised trials. BMJ 2017;358:j3064. [Crossref] [PubMed]
- Chia MC, Bilimoria KY. Benefits of Cluster Randomization for Surgical Trials and Quality Improvement. J Am Coll Surg 2020;231:400-2. [Crossref] [PubMed]
Cite this article as: Zhou C, Van der Hulst R. Alternative flaps for breast reconstruction: a narrative review on using the thigh, buttocks, and back. Ann Breast Surg 2023;7:19.

