
Breast cancer in the older population: a global challenge—an epidemiological perspective
In 2020, the estimated worldwide incidence of cancer has risen to 19.3 million cases per year (1). Breast cancer is the most commonly diagnosed malignancy among women, with more than 30% of all patients being over 70 years of age at the time of diagnosis (2). Due to the increasing incidence of breast cancer and ageing of the population, it is expected that the global number of new cases of women aged 70 years and older with breast cancer will have increased with at least 70% in 2040 (3,4). Despite this growing older population diagnosed with breast cancer, knowledge about possible differences in the biology and clinical breast cancer outcomes in this age group is limited. Unfortunately, older patients are still underrepresented in clinical trials and those older patients included in trials are generally fitter (5). This article provides an overview on several epidemiological aspects of breast cancer in older adults worldwide.
Ageing of the world populationOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Populations around the world are rapidly ageing, with the proportion of adults aged 65 years or older growing from 6% in 1990 to 9% in 2019 (6). This percentage is expected to increase to 16% in 2050, with the largest increase in Eastern and South-Eastern Asia (6). Ageing increases the exposure to age-related diseases, one of which is cancer (7). Therefore, by the age of 65, more than half of the population has multiple chronic diseases, which increases to over 80% in those aged 80 years and older (8). Currently, cancer is the second leading cause of death worldwide behind cardiovascular disease (9). However, in high-income countries, the mortality burden of cancer has surpassed that of cardiovascular disease, and it is expected that cancer will become the leading cause of morbidity and mortality worldwide (9). Therefore, we can expect a substantial increase in the number of older patients diagnosed with cancer, that will pose serious challenges to our health care system and resources.
Variation in breast cancer incidence between continentsOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
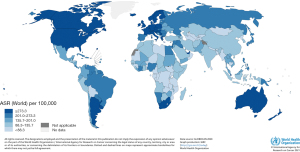
There is a global disparity in the incidence and mortality rate of breast cancer. Data from the Global Burden of Cancer study (GLOBOCAN) show that the incidence rates vary between and within continents. Northern America, Europe and Oceania generally have the highest estimated incidence rates, with age-standardized incidence rates of women of 70 years and older in 2020 of 394.6, 291.7, 372.3 per 100,000 persons, respectively (Figure 1) (4). Latin America with the Caribbean, Africa and Asia had age-standardized incidence rates of 215.4, 146.4 and 120.0 per 100,000 persons, respectively (Figure 1) (4). These high rates in the first three continents may be associated with intensive screening programmes and a higher prevalence of breast cancer risk factors, such as demographic, reproductive, hormonal and hereditary factors and lifestyle.
Breast cancer in older patientsOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Older women have more favourable biological tumour characteristics compared to younger patients with breast cancer. Tumours in the older population are more often hormone receptor-positive and HER2-negative (80% in patients aged ≥75 years and 65% in patients <50 years) and less often triple negative than tumours in younger patients (9% and 15% in patients aged ≥75 and <50 years, respectively) (10-12). Regarding the pathological type of malignancy, older women tend to have a higher rate of lobular carcinoma compared to younger patients (17% in patients aged ≥70 years and 2% among patients <40 years) (12,13). Older patients are also more likely to have less proliferating tumours (12). However, they are at higher risk to be diagnosed with more advanced tumours, and in a relatively large proportion of older women no proper staging is performed (10,14-16). The higher risk of advanced disease is thought to be due to delay in diagnosis at older age (17). These late diagnoses may partly be caused by decreasing self-awareness and reduced screening in the oldest old (18).
Breast cancer in aging patientsOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Ageing is a heterogeneous process, that differs for individuals. This results in a very heterogeneous older population with large variation in fitness and frailty. In general, as individuals age, body compositions change: fat mass increases while muscle tissue, bone density and function of organs decrease. These changes might affect or coincide with deterioration of other domains, such as functional status, physical activity, and cognition. Patients with a decline in one or more of these characteristics are often referred to as ‘frail’, which is a state of decreased physiologic reserve caused by the accumulation of ageing processes across multiple organ systems, which affects the patient’s resistance to stressors such as cancer or cancer therapy (19). A study investigating the prevalence of frailty among older patients with cancer classified 42% (range, 6–86%) as frail (20). Another study of women aged 65 years and older with breast cancer in the United States reported that more than 75% of patients were fit at time of diagnosis (21). Only 8% of older breast cancer patients in Australia were classified as frail in another study (22). Thus, it seems that the percentage of frailty is relatively small among older patients with breast cancer, but evidence remains scarce, and it might be due to under-reporting. Cancer and cognitive impairment are both age-related diseases, and therefore often coincide. Cognitive impairment may have significant consequences for older adults with breast cancer, impacting their level of independence, decision-making capacity, treatment compliance and quality of life. An American study showed that the prevalence of cognitive impairment in older patients with breast cancer was almost similar to age-matched controls without breast cancer (14% compared to 15%, respectively) (23). However, another study demonstrated that 41% of older patients with breast cancer had cognitive impairment at diagnosis, which is higher than what would have been expected in a comparable population without breast cancer (24,25). Moreover, a higher 10-year-cumulative-incidence of dementia was observed in older patients with breast cancer compared to their controls without breast cancer (hazard ratio 1.23; 95% confidence interval: 1.15–1.31; P value <0.001) (26). It is unclear whether this decline in cognition is due to cancer itself or to other factors, such as anticancer therapy (27). The effect of treatment on cognitive function has been excessively studied, but the results remain conflicting (28-31). Another aspect of older patients is the increase in functional dependence (32). Although multiple studies investigated functional status in patients with breast cancer, the variability among study populations, settings and questionnaires makes it difficult to interpret and compare these results with each other. One study showed that 51% of older patients had activity of daily living (ADL) impairment and 56% had instrumental ADL (IADL) impairment at the time of diagnosis (33). Another study of Hurria et al. investigated the effect of chemotherapy on physical function and demonstrated that 42% of older patients with breast cancer experienced a physical decline in the first month after initiation of chemotherapy. Interestingly, almost 50% of these patients recovered to approximately similar physical function scores at 12 months compared to baseline (34).
Screening programmesOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Although many countries have implemented population-based screening programmes to detect early breast cancer among asymptomatic women, several countries do not have formal screening programmes. Most programmes focus on women aged 50–69 years old, while some programmes have no upper age limit, a limit of 75 years or are based on life expectancy (35-39). It is arguable whether screening in this older age group is appropriate since it might lead to overtreatment (40,41). However, it could be beneficial in situations with sufficient health care and when the individual decision, risks and benefits, life expectancy and physiological age are taken into account (40). Importantly, it is not feasible nor cost-effective for every country to implement screening programmes. Especially developing countries may lack the resources, appropriate follow-up and treatment facilities to establish screening programmes and it is therefore not recommended (42).
TreatmentOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
The treatment of older patients is generally less aggressive than for their younger counterparts. This may partially explain the lack of improved relative survival of the former group (10). This difference in treatment strategies might be due to individual clinician preferences based on age, comorbidities or frailty (43). Due to frailty or comorbidity, intensive treatment such as chemotherapy, might not be feasible. In addition, older patients themselves might also have other treatment priorities, such as maintenance of quality of life and cognition or the ability to carry out daily tasks instead of prolongation of life (44). In addition, most guidelines do not provide specific guidance for the treatment of older patients with breast cancer. The International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA) did establish specific guidelines, which were recently revised (40,45). Some guidelines in other continents dedicate a small section to suggestions for treatment of older patients, but recommendations based on evidence-based medicine remain rare for this age group (46-48). Due to this lack of specific information, there is still a wide variation of treatment strategies within and between countries (49). The SIOG and EUSOMA state that the standard of care in surgical treatment for patients over 70 years with early-stage breast cancer should be similar to younger patients. Thus, either breast conserving surgery (BCS) combined with whole breast radiotherapy or mastectomy with or without postoperative radiotherapy (40,45). In general, older patients receive less surgical treatment, and less radiotherapy after BCS, compared to younger patients (10,50,51). The type of surgery and number of patients receiving radiation therapy also differ between countries and continents: BCS and radiotherapy is generally more common in Western Europe and the United States in comparison to Asian and African countries (13,49,52-57). There is also a large variety of patients receiving endocrine therapy and chemotherapy between different countries and age groups (10,52-54,58,59). These differences in patients receiving surgery, radiation therapy and (neo)adjuvant therapy are affected by many aspects, such as differences in socioeconomic status, cultural beliefs and accessibility and quality of health care (60-66). Although undertreatment is commonly reported among older women with breast cancer, it is difficult to discern how this impacts their prognosis and quality of life. Age alone should not dictate treatment decisions and it is therefore crucial to consider an individual’s life expectancy, preferences and the expected efficacy and the potential adverse effects of a treatment. It is therefore important to involve older patients in shared decision making, and to use tailored prediction models where possible (67). There are some prediction models available for older patients to guide clinical decision-making regarding treatment strategies, such as the PREDICT tool (68,69). It is important to use prediction tools who are also validated for the older population (70,71).
SurvivalOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
The age-standardized breast cancer mortality rate for women aged 70 years and older is highest in Europe with 118.0 deaths per 100,000 persons, followed by Oceania with 105.3 deaths per 100,000 persons. Asia and Latin America, including the Caribbean, have the lowest age-standardized mortality rates with 70.8 and 86.6 deaths per 100,000 persons, respectively (4). The difference between these continents regarding the mortality rate may be associated, among others, with the higher incidence rates in Northern America, Europe and Oceania. Furthermore, it is important to consider the contribution of competing causes of death when interpreting mortality rates; in lower-middle-income countries, a relatively large proportion of people will die due to other causes than breast cancer, such as infectious diseases or cardiovascular diseases (72). Moreover, the risk for older patients of dying of other causes than cancer is higher than for younger patients, with more than 50% of patients older than 75 years dying of other causes (16,73,74). The impact of these competing risks might increase in the older generation due to aging of the population. Therefore, survival estimates that account for competing risk of mortality are especially important for this age group. One way to estimate survival is by using Fine and Gray survival models that take other causes of death into account. However, it is difficult to obtain reliable information about cause of death in the older generation, therefore, estimating breast cancer-specific survival might be challenging. Another way to estimate survival rates is by using the relative survival as it takes into account the risk of dying from other causes, by dividing observed and expected survival based on the matched general population. Relative survival has improved in many countries for younger patients, but studies reported none or only a slight improvement for older patients (3,10,75-77). This might be due to different treatment strategies and the lack of clinical trials which provide evidence-based medicine for older adults with poorer health. Generally, developed countries have higher relative survival rates than developing countries, possibly because of a poor quality of cancer care with limited accessibility in many developing countries. The stage-standardized 5-year relative survival for older patients in high-income countries in Australia, Northern America and Northern and Western Europe is estimated to be above 85% (75,78,79). In the Eastern Mediterranean region, the 5-year relative survival for patients aged 65 and older was 58% (80). Studies in Asia showed a 5-year relative survival for this same age group of 66% in Singapore, while in a region in China this percentage was 41% for patients over 75 years of age (52,81). Korean women of 75 years and older had a 5-year relative survival of 65% (76). Africa showed similar varying rates with a 5-year relative survival of 41%, 67% and 87% for patients between 65–74 years of age in Zimbabwe, South Africa and Namibia, respectively (82). Nevertheless, it remains questionable whether cancer statistics of different countries or continents can be compared to each other, because of inconsistency in data collection and differences in the reliability of reported numbers.
Role of geriatric screening and assessment in treatment decisions and prognosisOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Chronological age can differ from biological age and therefore is a poor indicator of the physiological and functional status of older adults. However, chronological age is often used in medical decision making, which can lead to both under- and overtreatment (43,83). Currently, the SIOG and EUSOMA advice a geriatric screening tool, such as the G8, for all patients aged 70 years and older to distinguish patients in either fit or potentially frail patients (40,84). If potentially frail, a comprehensive geriatric assessment should be performed to get a grip on an individual’s fitness and frailty. A comprehensive geriatric assessment is a multidisciplinary evaluation that provides information about several domains, such as comorbidities, medication use, functional status, physical function, cognition, emotion, nutrition and psychosocial status. An important reason to perform a comprehensive geriatric assessment is to detect unidentified problems and risks to guide integrated geriatric and supportive care interventions (85,86). Another reason for performing a geriatric assessment is the ability to get a better impression about possible treatment outcomes, such as side effects (85,87). A comprehensive geriatric assessment also gives a better estimation of the expected life expectancy taking the competing risks into account (85). Despite the evidence of the beneficial effect of geriatric assessments regarding decision-making, treatment outcomes and treatment adherence, their current use in daily practise is still limited (88,89). This might be due to the inability to interpret a geriatric assessment (90). Moreover, a geriatric assessment is time consuming. There are also other interesting tools specifically designed to predict treatment outcomes, such as the chemotherapy toxicity tool developed by Hurria et al. on chemotoxicity in patients with cancer (87,91).
Performing research in an older population with breast cancerOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Most clinical trials are based on younger patients and those older patients participating in trials often constitute a relatively healthy, homogeneous group (92-94). Conversely, as stated before, the general population of patients of 70 years and older is very heterogeneous regarding fitness, cognitive function and socioeconomic status. Consequently, this discrepancy between the general population and those included in clinical trial makes it challenging for clinicians to discern whether the study results can be extrapolated to clinical practice (95,96). Although several organizations encouraged researchers to design more trials for older patients, only 2% of all ongoing clinical trials on breast cancer treatment are specifically designed for older patients (94,97,98). As current clinical trials will not improve treatment strategies for older patients in the next decades, alternative research methods should be considered. An acceptable substitute could be the use of observational cohort studies when appropriate methodological methods to tackle confounding by indication, and outcomes are used (99). Currently, the most frequently reported outcomes are cancer-related endpoints, such as disease-free survival or overall survival (94). It remains questionable whether these endpoints are adequate and relevant to determine an appropriate risk-benefit ratio of therapy for older patients (44). Not only because older patients have a higher risk of competing mortality, but also because they may prefer maintenance of quality of life and functional status over prolongation of life (44). Future research should focus on older patients with relevant endpoints, not only in developed countries, but also in less developed countries.
ConclusionsOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Breast cancer is the most common cancer among women, affecting an increasing number of older adults around the world. Older patients with breast cancer comprise a very heterogeneous group, with differences in fitness and frailty. Chronological age alone should not be the basis of shared decision making, but rather an individual’s life expectancy, preferences and the expected efficacy and the potential adverse effects of a treatment. It is therefore recommended to implement a screening tool for older patients to distinguish fit from frail patients and improve personalized medicine in the older population.
AcknowledgmentsOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Funding: None.
FootnoteOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kwok-Leung Cheung) for the series “Diagnosis and Treatment on Primary Breast Cancer in Older Women” published in Annals of Breast Surgery. The article has undergone external peer review.
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-89/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-89/coif). The series “Diagnosis and Treatment on Primary Breast Cancer in Older Women” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Ageing of the world population
- Variation in breast cancer incidence between continents
- Breast cancer in older patients
- Breast cancer in aging patients
- Screening programmes
- Treatment
- Survival
- Role of geriatric screening and assessment in treatment decisions and prognosis
- Performing research in an older population with breast cancer
- Conclusions
- Acknowledgments
- Footnote
- References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019;69:438-51. [Crossref] [PubMed]
- Hu K, Ding P, Wu Y, et al. Global patterns and trends in the breast cancer incidence and mortality according to sociodemographic indices: an observational study based on the global burden of diseases. BMJ Open 2019;9:e028461. [Crossref] [PubMed]
- Arnold M RM, Lam F, Bray F, Ervik M, Soerjomataram I ICBP SURVMARK-2 online tool: International Cancer Survival Benchmarking. Lyon, France: International Agency for Research on Cancer. 2019. Available online: https://gco.iarc.fr/survival/survmark/. Accessed [22/06/2021].
- Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 2003;21:1383-9. [Crossref] [PubMed]
- United Nations DoEaSA, Population Division. World Population Ageing 2019: Hightlights, 2019.
- White MC, Holman DM, Boehm JE, et al. Age and cancer risk: a potentially modifiable relationship. Am J Prev Med 2014;46:S7-15. [Crossref] [PubMed]
- Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. [Crossref] [PubMed]
- Dagenais GR, Leong DP, Rangarajan S, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet 2020;395:785-94. [Crossref] [PubMed]
- de Glas N, Bastiaannet E, de Boer A, et al. Improved survival of older patients with advanced breast cancer due to an increase in systemic treatments: a population-based study. Breast Cancer Res Treat 2019;178:141-9. [Crossref] [PubMed]
- Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106:dju055. [Crossref] [PubMed]
- Durbecq V, Ameye L, Veys I, et al. A significant proportion of elderly patients develop hormone-dependant "luminal-B" tumours associated with aggressive characteristics. Crit Rev Oncol Hematol 2008;67:80-92. [Crossref] [PubMed]
- Ma CD, Zhou Q, Nie XQ, et al. Breast cancer in Chinese elderly women: pathological and clinical characteristics and factors influencing treatment patterns. Crit Rev Oncol Hematol 2009;71:258-65. [Crossref] [PubMed]
- Grumpelt AM, Ignatov A, Tchaikovski SN, et al. Tumor characteristics and therapy of elderly patients with breast cancer. J Cancer Res Clin Oncol 2016;142:1109-16. [Crossref] [PubMed]
- Louwman WJ, Vulto JC, Verhoeven RH, et al. Clinical epidemiology of breast cancer in the elderly. Eur J Cancer 2007;43:2242-52. [Crossref] [PubMed]
- Bastiaannet E, Liefers GJ, de Craen AJ, et al. Breast cancer in elderly compared to younger patients in the Netherlands: stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res Treat 2010;124:801-7. [Crossref] [PubMed]
- DeSantis CE, Miller KD, Dale W, et al. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin 2019;69:452-67. [Crossref] [PubMed]
- Arndt V, Stürmer T, Stegmaier C, et al. Patient delay and stage of diagnosis among breast cancer patients in Germany -- a population based study. Br J Cancer 2002;86:1034-40. [Crossref] [PubMed]
- Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: implications for clinical practice and public health. Lancet 2019;394:1365-75. [Crossref] [PubMed]
- Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol 2015;26:1091-101. [Crossref] [PubMed]
- Sheppard VB, Faul LA, Luta G, et al. Frailty and adherence to adjuvant hormonal therapy in older women with breast cancer: CALGB protocol 369901. J Clin Oncol 2014;32:2318-27. [Crossref] [PubMed]
- To THM, Okera M, Prouse J, et al. Infancy of an Australian geriatric oncology program—characteristics of the first 200 patients. J Geriatr Oncol 2010;1:81-6. [Crossref]
- Mandelblatt JS, Stern RA, Luta G, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J Clin Oncol 2014;32:1909-18. [Crossref] [PubMed]
- Lange M, Giffard B, Noal S, et al. Baseline cognitive functions among elderly patients with localised breast cancer. Eur J Cancer 2014;50:2181-9. [Crossref] [PubMed]
- Dijkshoorn ABC, van Stralen HE, Sloots M, et al. Prevalence of cognitive impairment and change in patients with breast cancer: A systematic review of longitudinal studies. Psychooncology 2021;30:635-48. [Crossref] [PubMed]
- Roderburg C, Loosen SH, Kunstein A, et al. Cancer Patients Have an Increased Incidence of Dementia: A Retrospective Cohort Study of 185,736 Outpatients in Germany. Cancers (Basel) 2021;13:2027. [Crossref] [PubMed]
- Bai L, Yu E. A narrative review of risk factors and interventions for cancer-related cognitive impairment. Ann Transl Med 2021;9:72. [Crossref] [PubMed]
- Hurria A, Goldfarb S, Rosen C, et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient's perspective. Breast Cancer Res Treat 2006;98:343-8. [Crossref] [PubMed]
- Freedman RA, Pitcher B, Keating NL, et al. Cognitive function in older women with breast cancer treated with standard chemotherapy and capecitabine on Cancer and Leukemia Group B 49907. Breast Cancer Res Treat 2013;139:607-16. [Crossref] [PubMed]
- Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol 2010;28:4434-40. [Crossref] [PubMed]
- Schilder CM, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol 2010;28:1294-300. [Crossref] [PubMed]
- Lavelle K, Moran A, Howell A, et al. Older women with operable breast cancer are less likely to have surgery. Br J Surg 2007;94:1209-15. [Crossref] [PubMed]
- Deckx L, van den Akker M, Daniels L, et al. Geriatric screening tools are of limited value to predict decline in functional status and quality of life: results of a cohort study. BMC Fam Pract 2015;16:30. [Crossref] [PubMed]
- Hurria A, Soto-Perez-de-Celis E, Allred JB, et al. Functional Decline and Resilience in Older Women Receiving Adjuvant Chemotherapy for Breast Cancer. J Am Geriatr Soc 2019;67:920-7. [Crossref] [PubMed]
- Youlden DR, Cramb SM, Dunn NA, et al. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 2012;36:237-48. [Crossref] [PubMed]
- Green M, Raina V. Epidemiology, screening and diagnosis of breast cancer in the Asia–Pacific region: Current perspectives and important considerations. Asia Pac J Clin Oncol 2008;4:5-13. [Crossref]
- Schünemann HJ, Lerda D, Quinn C, et al. Breast Cancer Screening and Diagnosis: A Synopsis of the European Breast Guidelines. Ann Intern Med 2020;172:46-56. [Crossref] [PubMed]
- Williams J, Garvican L, Tosteson AN, et al. Breast cancer screening in England and the United States: a comparison of provision and utilisation. Int J Public Health 2015;60:881-90. [Crossref] [PubMed]
- Sitt JC, Lui CY, Sinn LH, et al. Understanding breast cancer screening--past, present, and future. Hong Kong Med J 2018;24:166-74. [Crossref] [PubMed]
- Biganzoli L, Battisti NML, Wildiers H, et al. Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol 2021;22:e327-40. [Crossref] [PubMed]
- de Glas NA, de Craen AJ, Bastiaannet E, et al. Effect of implementation of the mass breast cancer screening programme in older women in the Netherlands: population based study. BMJ 2014;349:g5410. [Crossref] [PubMed]
- Ba DM, Ssentongo P, Agbese E, et al. Prevalence and determinants of breast cancer screening in four sub-Saharan African countries: a population-based study. BMJ Open 2020;10:e039464. [Crossref] [PubMed]
- Lavelle K, Sowerbutts AM, Bundred N, et al. Is lack of surgery for older breast cancer patients in the UK explained by patient choice or poor health? A prospective cohort study. Br J Cancer 2014;110:573-83. [Crossref] [PubMed]
- Wildiers H, Mauer M, Pallis A, et al. End points and trial design in geriatric oncology research: a joint European organisation for research and treatment of cancer--Alliance for Clinical Trials in Oncology--International Society Of Geriatric Oncology position article. J Clin Oncol 2013;31:3711-8. [Crossref] [PubMed]
- Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol 2012;13:e148-60. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J Natl Compr Canc Netw 2017;15:433-51. [Crossref] [PubMed]
- Park YH, Senkus-Konefka E, Im SA, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with early breast cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol 2020;31:451-69. [Crossref] [PubMed]
- Cancer Australia. Guidance for the management of early breast cancer: Recommendations and practice points. 2020.
- Derks MGM, Bastiaannet E, Kiderlen M, et al. Variation in treatment and survival of older patients with non-metastatic breast cancer in five European countries: a population-based cohort study from the EURECCA Breast Cancer Group. Br J Cancer 2018;119:121-9. [Crossref] [PubMed]
- Yin M, Verschraegen C, Vincent VH, et al. Impact of lack of surgery on outcomes in elderly women with nonmetastatic breast cancer-A surveillance, epidemiology, and end results 18 population based study. Medicine (Baltimore) 2020;99:e18745. [Crossref] [PubMed]
- Hamaker ME, Bastiaannet E, Evers D, et al. Omission of surgery in elderly patients with early stage breast cancer. Eur J Cancer 2013;49:545-52. [Crossref] [PubMed]
- Saxena N, Hartman M, Hussain Z, et al. Impact of older age on presentation, management and outcome of breast cancer in the multi-ethnic Asian population of Singapore. J Geriatr Oncol 2011;2:50-7. [Crossref]
- Liu X, Zheng D, Wu Y, et al. Treatment patterns and outcomes in older women with early breast cancer: a population-based cohort study in China. BMC Cancer 2021;21:226. [Crossref] [PubMed]
- Jeon YW, You SH, Lee JE, et al. Optimal treatment of breast cancer in women older than 75 years: a Korea Breast Cancer Registry analysis. Breast Cancer Res Treat 2019;178:693-701. [Crossref] [PubMed]
- Kiderlen M, Bastiaannet E, Walsh PM, et al. Surgical treatment of early stage breast cancer in elderly: an international comparison. Breast Cancer Res Treat 2012;132:675-82. [Crossref] [PubMed]
- Leong SP, Shen ZZ, Liu TJ, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg 2010;34:2308-24. [Crossref] [PubMed]
- Cubasch H, Joffe M, Ruff P, et al. Breast conservation surgery versus total mastectomy among women with localized breast cancer in Soweto, South Africa. PLoS One 2017;12:e0182125. [Crossref] [PubMed]
- Schuil H, Derks M, Liefers GJ, et al. Treatment strategies and survival outcomes in older women with breast cancer: A comparative study between the FOCUS cohort and Nottingham cohort. J Geriatr Oncol 2018;9:635-41. [Crossref] [PubMed]
- Kiderlen M, Walsh PM, Bastiaannet E, et al. Treatment strategies and survival of older breast cancer patients - an international comparison between the Netherlands and Ireland. PLoS One 2015;10:e0118074. [Crossref] [PubMed]
- Chabba N, Tin ST, Zhao J, et al. Geographic variations in surgical treatment for breast cancer: a systematic review. Ann Cancer Epidemiol 2020;4:2. [Crossref]
- Gu J, Groot G, Boden C, et al. Review of Factors Influencing Women's Choice of Mastectomy Versus Breast Conserving Therapy in Early Stage Breast Cancer: A Systematic Review. Clin Breast Cancer 2018;18:e539-54. [Crossref] [PubMed]
- Ruff P, Al-Sukhun S, Blanchard C, et al. Access to Cancer Therapeutics in Low- and Middle-Income Countries. Am Soc Clin Oncol Educ Book 2016;35:58-65. [Crossref] [PubMed]
- Hossain MS, Ferdous S, Karim-Kos HE. Breast cancer in South Asia: a Bangladeshi perspective. Cancer Epidemiol 2014;38:465-70. [Crossref] [PubMed]
- Zubizarreta EH, Fidarova E, Healy B, et al. Need for radiotherapy in low and middle income countries – the silent crisis continues. Clin Oncol (R Coll Radiol) 2015;27:107-14. [Crossref] [PubMed]
- Anderson BO, Cazap E, El Saghir NS, et al. Optimisation of breast cancer management in low-resource and middle-resource countries: executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol 2011;12:387-98. [Crossref] [PubMed]
- Fan L, Goss PE, Strasser-Weippl K. Current Status and Future Projections of Breast Cancer in Asia. Breast Care (Basel) 2015;10:372-8. [Crossref] [PubMed]
- Hamelinck VC, Bastiaannet E, Pieterse AH, et al. Preferred and Perceived Participation of Younger and Older Patients in Decision Making About Treatment for Early Breast Cancer: A Prospective Study. Clin Breast Cancer 2018;18:e245-53. [Crossref] [PubMed]
- Wishart GC, Azzato EM, Greenberg DC, et al. PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res 2010;12:R1. [Crossref] [PubMed]
- de Glas NA, Bastiaannet E, Engels CC, et al. Validity of the online PREDICT tool in older patients with breast cancer: a population-based study. Br J Cancer 2016;114:395-400. [Crossref] [PubMed]
- de Glas NA, van de Water W, Engelhardt EG, et al. Validity of Adjuvant! Online program in older patients with breast cancer: a population-based study. Lancet Oncol 2014;15:722-9. [Crossref] [PubMed]
- Engelhardt EG, Garvelink MM, de Haes JH, et al. Predicting and communicating the risk of recurrence and death in women with early-stage breast cancer: a systematic review of risk prediction models. J Clin Oncol 2014;32:238-50. [Crossref] [PubMed]
- World Health Organization (WHO). The top 10 causes of death. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- Chen HL, Zhou MQ, Tian W, et al. Effect of Age on Breast Cancer Patient Prognoses: A Population-Based Study Using the SEER 18 Database. PLoS One 2016;11:e0165409. [Crossref] [PubMed]
- Derks MGM, van de Velde CJH, Giardiello D, et al. Impact of Comorbidities and Age on Cause-Specific Mortality in Postmenopausal Patients with Breast Cancer. Oncologist 2019;24:e467-74. [Crossref] [PubMed]
- Nordenskjöld AE, Fohlin H, Arnesson LG, et al. Breast cancer survival trends in different stages and age groups - a population-based study 1989-2013. Acta Oncol 2019;58:45-51. [Crossref] [PubMed]
- Lee JY, Jung KW, Park S, et al. Long-term survival of cancer patients in Korea, 1993-2007: National Cancer Registry Study. Asian Pac J Cancer Prev 2010;11:1459-64. [PubMed]
- Smith BD, Jiang J, McLaughlin SS, et al. Improvement in breast cancer outcomes over time: are older women missing out? J Clin Oncol 2011;29:4647-53. [Crossref] [PubMed]
- Jansen L, Holleczek B, Kraywinkel K, et al. Divergent Patterns and Trends in Breast Cancer Incidence, Mortality and Survival Among Older Women in Germany and the United States. Cancers (Basel) 2020;12:2419. [Crossref] [PubMed]
- Western Australia Cancer Registry and the Epidemiology Branch WA Department of Health. The Cancer Effect: An "Exploring Cancer" Series Western Australia. Breast Cancer Relative Survival 1985-2014. 2018. Available online: https://ww2.health.wa.gov.au/~/media/Files/Corporate/Reports-and-publications/The-cancer-effect/The-Cancer-Effect-Breast-Cancer.pdf
- Maajani K, Khodadost M, Fattahi A, et al. Survival rates of patients with breast cancer in countries in the Eastern Mediterranean Region: a systematic review and meta-analysis. East Mediterr Health J 2020;26:219-32. [Crossref] [PubMed]
- Yan X, Han R, Zhou J, et al. Incidence, mortality and survival of female breast cancer during 2003-2011 in Jiangsu province, China. Chin J Cancer Res 2016;28:321-9. [Crossref] [PubMed]
- Joko-Fru WY, Miranda-Filho A, Soerjomataram I, et al. Breast cancer survival in sub-Saharan Africa by age, stage at diagnosis and human development index: A population-based registry study. Int J Cancer 2020;146:1208-18. [Crossref] [PubMed]
- Hurria A, Wong FL, Villaluna D, et al. Role of age and health in treatment recommendations for older adults with breast cancer: the perspective of oncologists and primary care providers. J Clin Oncol 2008;26:5386-92. [Crossref] [PubMed]
- Bellera CA, Rainfray M, Mathoulin-Pélissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol 2012;23:2166-72. [Crossref] [PubMed]
- Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595-603. [Crossref] [PubMed]
- Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018;36:2326-47. [Crossref] [PubMed]
- Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457-65. [Crossref] [PubMed]
- Dale W, Williams GR, R, MacKenzie A, et al. How Is Geriatric Assessment Used in Clinical Practice for Older Adults With Cancer? A Survey of Cancer Providers by the American Society of Clinical Oncology. JCO Oncol Pract 2021;17:336-44. [Crossref] [PubMed]
- Hamaker ME, Te Molder M, Thielen N, et al. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients - A systematic review. J Geriatr Oncol 2018;9:430-40. [Crossref] [PubMed]
- Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric Assessment and Tools for Predicting Treatment Toxicity in Older Adults With Cancer. Cancer J 2017;23:206-10. [PubMed]
- Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol 2016;34:2366-71. [Crossref] [PubMed]
- Ludmir EB, Mainwaring W, Lin TA, et al. Factors Associated With Age Disparities Among Cancer Clinical Trial Participants. JAMA Oncol 2019;5:1769-73. [Crossref] [PubMed]
- Kornblith AB, Kemeny M, Peterson BL, et al. Survey of oncologists' perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer 2002;95:989-96. [Crossref] [PubMed]
- de Glas NA, Hamaker ME, Kiderlen M, et al. Choosing relevant endpoints for older breast cancer patients in clinical trials: an overview of all current clinical trials on breast cancer treatment. Breast Cancer Res Treat 2014;146:591-7. [Crossref] [PubMed]
- Singh H, Beaver JA, Kim G, et al. Enrollment of older adults on oncology trials: An FDA perspective. J Geriatr Oncol 2017;8:149-50. [Crossref] [PubMed]
- van de Water W, Kiderlen M, Bastiaannet E, et al. External validity of a trial comprised of elderly patients with hormone receptor-positive breast cancer. J Natl Cancer Inst 2014;106:dju051. [Crossref] [PubMed]
- Levit LA, Singh H, Klepin HD, et al. Expanding the Evidence Base in Geriatric Oncology: Action Items From an FDA-ASCO Workshop. J Natl Cancer Inst 2018;110:1163-70. [Crossref] [PubMed]
- Extermann M, Brain E, Canin B, et al. Priorities for the global advancement of care for older adults with cancer: an update of the International Society of Geriatric Oncology Priorities Initiative. Lancet Oncol 2021;22:e29-36. [Crossref] [PubMed]
- de Glas NA, Kiderlen M, de Craen AJ, et al. Assessing treatment effects in older breast cancer patients: systematic review of observational research methods. Cancer Treat Rev 2015;41:254-61. [Crossref] [PubMed]
Cite this article as: Lemij AA, Bastiaannet E, de Glas NA, van den Bos F, Portielje JEA, Liefers GJ, Derks MGM. Breast cancer in the older population: a global challenge—an epidemiological perspective. Ann Breast Surg 2023;7:17.

