An observational study comparing the SPY-Elite® vs. the SPY-PHI QP system in breast reconstructive surgery
Introduction
Imaging techniques visualizing the per-operative tissue perfusion has been used in plastic surgery for more than 2 decades (1-3). A well-known imaging modality is indocyanine green angiography (ICG-A) which can assist the surgeon in the intraoperative decision making by supplying information on real-time tissue perfusion (4,5). Recent studies have shown that the action taking upon applying ICG-A in breast reconstruction can lead to a decreased risk of postoperative complications including loss of reconstruction (6-9).
Multiple imaging systems are available including the PicoLinker wearable smartglasses (Westunitis Co., Ltd., Osaka, Japan) (10), Fluobeam Clinical System® (Fluoptics, Grenoble, France, https://www.fluoptics.com/), HyperEye Medical Systems® (Mizuho, Tokyo, Japan, http://www.mizuhomedical.co.jp/) and the SPY-Elite® Fluorescence Imaging System/SPY-PHI (Stryker AB, Malmö, Sweden, https://www.stryker.com/) (11).
Two different imaging modalities encompass the capability to assess and quantify the relative perfusion values, namely the SPY-Elite imaging system® and the SPY-PHI QP system (Stryker AB, Malmö, Sweden, https://www.stryker.com/).
The SPY-Elite Fluorescence imaging system®, which is one of the most commonly used systems, contains the SPY-Q-software which is able to quantify the relative perfusion and has been studied by several authors (9,12-15). Based on this system, Moyer et al. reported a cut-off value of 33% corresponding with a positive predictive value (PPV) of 88% and a negative predictive value (NPV) of 16% for mastectomy flap necrosis (15).
The SPY-QP® Fluorescence assessment software is an upgrade of the existing SPY Portable Handheld Imaging (SPY-PHI) system, and is the most recent fluorescence assessment technique with software able to quantify the relative perfusion (16). This upgrade, the SPY-PHI QP, consists of integrated software able to assess and quantify the relative tissue perfusion.
The SPY-QP® and the SPY-PHI, combined called SPY-PHI QP, is based on near-infrared (NIR) fluorescence that enables relative quantification of perfusion upon the per-operative intravenous injection of indocyanine green (ICG) (17).
As this technology is constantly evolving, how does the surgeon interpret the per-operative data on the quantitative perfusion?
May the two imaging modalities and quantification systems be equated? How can we translate the relative perfusion data into clinical decision making?
The purpose of this study was to compare the relative tissue perfusion and the quantitative values of the SPY-PHI QP and the SPY-Elite®. Secondly to investigate if the per-operative assessment of the perfusion corresponded to the postoperative events evaluated at 4-week follow-up. We present the following article in accordance with the STARD reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-123/rc).
Methods
Patients
Sixteen consecutive patients undergoing either an immediate or delayed breast reconstructive procedure (including breast conserving surgery (BCS), implant-based- or autologous breast reconstruction using pedicled- or free flaps [deep inferior epigastric artery perforator flaps (DIEP-flaps) and latissimus dorsi muscle flaps (LD-flaps)].
Patients were included prospectively from March to April 2021 at the Department of Plastic Surgery and Burns Treatment, Copenhagen University Hospital.
Patient demographics are depicted in Table 1. Inclusion criteria were: patient >18 years of age and deemed suitable for a breast reconstructive procedure by the consultant plastic- and breast surgeon. Patients allergic to iodine, pregnant or breastfeeding were excluded.
Table 1
| Characteristics | Values |
|---|---|
| No. of patients | 16 |
| Age (mean), years | 52 |
| BMI (mean), kg/m2 | 24.8 |
| Immediate breast reconstruction, n | 7 |
| Delayed breast reconstruction, n | 5 |
| Oncoplastic procedure, n | 4 |
| Implant-based reconstruction, n | 5 |
| Autologous reconstruction, n | 7 |
BMI, body mass index.
Data collection
This is an observational study of two imaging modalities already approved for clinical use. There has not been any intervention. Therefore, ethical approval was not required. All data was obtained and stored anonymously. Inform consent was not required. This observational study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
ICG-A methodology
We applied the SPY-PHI QP and the SPY-Elite imaging system® per-operatively on oncoplastic and breast reconstructive procedures, and compared the relative perfusion values of both imaging systems with postoperative complications evaluated 4-week after surgery.
In implant-based immediate breast reconstruction per-operative ICG-A was performed subsequent to mastectomy upon insertion of an appropriate sizer and after temporary closure of dermis. For the oncoplastic procedures, ICG-A was performed after excision of pathological tissue, before reshaping the breast with replacement- or displacement techniques.
In the autologous reconstructions (pedicled- and free flaps), ICG-A was performed after the flap was raised with complete pedicle dissection and still located at the donor-site.
Regardless of the chosen procedure, an intravenous bolus administration of ICG (VerdyeÒ 5 mg/mL) of 2.5 mg/mL was followed by a 10 ml flush with normal saline. The surgeon refrained from using vasoconstrictive agents (i.e., Klein’s fluid-Ringer’s lactate, lidocaine and adrenaline) avoiding potential bias of the ICG-A assessment.
The SPY-Elite® and the SPY-PHI QP system were applied simultaneously, and perfusion values scored and quantified by the same operator. Quantification of the relative values was done after 45 seconds of recording (15).
Adjacent healthy tissue outside the surgical field was used as reference point (100% perfusion).
Both imaging modalities were thoroughly tested before initiating the study, minimizing possible user error and device learning curve.
Cut-off perfusion value
When assessing tissue perfusion, the surgeon relies on clinical evaluation which may be supplemented using an imaging modality such as the SPY-PHI QP® or the SPY-Elite imaging system®. ICG-A showing hypoperfusion has been reported to correlate with postoperative complications, making accurate and reliable intraoperative assessment an important factor in identifying areas in risk of potential hypoperfusion (9,14,18-21).
To make the per-operative assessment of the relative tissue perfusion and quantification clinically relevant, there is a need for a cut-off value.
Though investigated by several authors, determining specific cut-off for relative perfusion values remains to be identified (12-15,22-24). For mastectomy skin flap necrosis, Moyer et al. suggested a cut-off value of 33% with a PPV of 88% and NPV of 16% (15).
There has not been reported a specific cut-off value for autologous breast reconstruction (6,18,25-28). We used the cut-off value of 33% as described by Moyer et al. (15).
Quantification using the SPY-Elite® software
The SPY-Elite® camera is positioned 20 cm perpendicular to the tissue of interest. All light in the OR turned off, and recording of the angiography activated subsequent to injection of ICG and flush with saline. Recording was terminated after reaching 60 seconds. Quantification of the relative perfusion was performed after 45 seconds of recording (15).
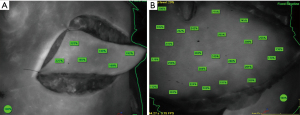
For the implant-based breast reconstruction, the breast was divided into 4 quadrants (upper lateral, lower lateral, upper medial and lower medial quadrant). Quantification marks (%) were set in each quadrant and an average perfusion quantification (%) calculated resulting in 4 relative perfusion values (%) for each breast (Figure 1).

For the oncoplastic procedures and autologous breast reconstruction, relative perfusion marks were set in the circumference and across the surface, assessing the perfusion of the entire flap (Figure 2).
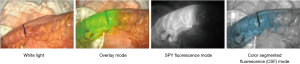
Quantification using the SPY-PHI QP software
This system contains 4 different visualization modes (4 fluorescence imaging modalities) (Figure 3) (16). We applied the SPY contrast mode in order to compare the angiographies and to assess the relative perfusion values (% tissue perfusion).
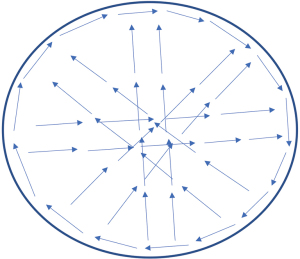
After activating the SPY-PHI QP system, the handheld camera is positioned 10–40 cm above the tissue of interest. Detection of fluorescence initiates an automatic timer on the SPY-PHI QP monitor and a red icon appears to signal onset of fluorescence.
Relative values (% tissue perfusion) can be assessed when the fluorescence signal has stabilized and a green icon appears on the monitor. Quantification mode is enabled, and a small square with a relative number and percentage will appear. To obtain relative tissue perfusion, the cursor is set at a reference point (100% perfusion) and the handheld camera is then positioned back above the tissue of interest.
Video 1 shows how the SPY-PHI QP system was applied in implant-based breast reconstructions. For the autologous breast reconstructions, the SPY-PHI QP was applied in a clock wise manner and crossing the tissue in all directions encompassing the total area of the flap (Figure 4, Videos 2,3).
Endpoints
Our primary endpoint was to compare the per-operative quantitative perfusion values of both imaging systems. The clinical endpoints were incidence of per-and postoperative complications (including mastectomy skin flap necrosis, flap necrosis, fat necrosis and loss of reconstruction). Major complications were defined as complications requiring surgery/debridement in local- or general anesthesia. Minor complications were defined as complications treated conservatively (seroma, small hematoma etc.). All patients were followed up 4 weeks after breast reconstruction.
Statistical analysis
Perfusion measurements of the two imaging modalities were performed and compared by calculating sensitivity, specificity, PPV, NPV and accuracies.
Results
Sixteen consecutive patients (20 breasts) were included. Mean age was 52 years (range, 35–74 years). Mean body mass index (BMI) was 24.8 kg/m2 (range, 19.1–29.6 kg/m2). One patient was an active smoker until the day of surgery. Seven patients had immediate breast reconstruction, 5 delayed reconstruction and 4 underwent oncoplastic surgeries. Autologous reconstructions constituted 7/16 patients (5 DIEP-flaps and 2 LD-flaps). Radiation was the most frequent adjuvant therapy (9/16 patients) (Table 1). The surgical indication was predominantly breast cancer, with 12/16 patients having sentinel node biopsy performed during the oncologic and reconstructive surgery.
Thirteen patients had uneventful healing within the 4-week follow-up. All patients attained reconstructive surgery of the breast and there was no loss of reconstruction.
At 4-week follow up, 3 patients had experienced complications (Table 2, Case no. 2, 10 and 12). Case no. 2 developed bilateral full thickness peri incisional skin necroses treated with debridement in local anesthesia, she also had a hematoma evacuated by ultrasound. Case no. 10 developed seroma on both breasts and on the donor-site, treated by ultrasound guided drainage. Case no. 10 also developed unilateral skin necrosis treated conservatively. One patient (Case no. 12) developed a postoperative pulmonary embolism (PE), melaena and gastritis. She was treated with standard protocols.
Table 2
| Case no. | Type of breast reconstruction | I/D | U/B | SPY-Elite® quantification | SPY-PHI QP quantification | Equal quantification? | Eventful healing | Complications |
|---|---|---|---|---|---|---|---|---|
| 1 | Implant-based | I | U | >33% | 15–17% | No | − | None |
| 2 | Implant-based | I | B | Area with hypoperfusion <33% | Area with hypoperfusion 5–10% | No | + | Breast hematoma, bilat. + bilat. skin necrosis |
| 3 | Implant-based | I | B | >33% | <33% | No | − | None |
| 4 | Implant-based | I | U | >33% | >33% | Yes | − | None |
| 5 | Implant-based | I | B | >33% | >33% | Yes | − | None |
| 6 | Oncoplasty D | I | U | >33% | >33% | Yes | − | None |
| 7 | Oncoplasty R | I | U | 88–104% | 28–34% | No | − | None |
| 8 | Oncoplasty D | I | U | >33% | >33% | Yes | − | None |
| 9 | LD-flap | D | U | >33% | >33% | Yes | − | None |
| 10 | LD-flap | I | B | >33% | >33% | Yes | + | Hematoma bilat. breast + back + skin necrosis left breast |
| 11 | Oncoplasty R | I | U | >33% | >33% | Yes | − | None |
| 12 | DIEP-flap | D | U | >33% | >33% | Yes | + | Postop. PE + melaena* |
| 13 | DIEP-flap | D | U | >33% | >33% | Yes | − | None |
| 14 | DIEP-flap | I | U | >33% | >33% | Yes | − | None |
| 15 | DIEP-flap | D | U | >33% | >33% | Yes | − | None |
| 16 | DIEP-flap | D | U | >33% | >33% | Yes | − | None |
Total no. of breast: 20. Cases organized by type of surgery. *, melaena due to gastritis. I/D, immediate breast reconstruction, delayed breast reconstruction; U/B, unilateral, bilateral; oncoplasty D/R, D = volume displacement technique, R = volume replacement technique; DIEP-flap, deep inferior epigastric artery perforator flap; LD-flap, latissimus dorsi muscle flap; postop. PE, postoperative pulmonal embolism.
Total incidence of complications per breast were 2 major complications and 5 minor complications. Case no. 12 was excluded due to postoperative complications not related to the relative tissue perfusion of the breast.
The quantitative perfusion values of the SPY-Elite® was compared with the SPY-PHI QP system (Table 2). Measurements were not equal in 4/16 cases (25%), with the SPY-PHI QP quantification being substantially lower than the SPY-Elite®.
One of the 4 cases (25%) where the quantification systems were unequal, developed major complications (Case no. 2, Table 2).
There exists no gold standard to investigate per-operative tissue perfusion. To compare the perfusion measurements of the two modalities; sensitivity, specificity, PPV, NPV and accuracies were calculated.
Using a quantitative cut-off value of 33%, the SPY-PHI QP yielded a sensitivity of 50%, specificity 77%, PPV 25%, NPV 91% and accuracy of 73% (Table 3). The SPY-Elite® had a sensitivity of 50%, specificity 100%, PPV 100%, NPV 93% and accuracy of 93% (Table 4).
Table 3
| SPY-PHI QP quantification | Eventful healing | Uneventful healing | Number of patients |
|---|---|---|---|
| SPY-QP perfusion <33% | 1 | 3 | 4 |
| SPY-QP perfusion >33% | 1 | 10 | 11 |
| Total | 2 | 13 | 15 |
Sensitivity: 0.50; specificity: 0.77; PPV: 0.25; NPV: 0.91; accuracy: 0.73. Case no. 12 excluded. PPV, positive predictive value; NPV, negative predictive value.
Table 4
| SPY-Elite® quantification | Eventful healing | Uneventful healing | Number of patients |
|---|---|---|---|
| SPY-Elite® perfusion <33% | 1 | 0 | 1 |
| SPY-Elite® perfusion >33% | 1 | 13 | 14 |
| Total | 2 | 13 | 15 |
Sensitivity: 0.50; specificity: 1; PPV: 1; NPV: 0.93; accuracy: 0.93. Case no. 12 excluded. PPV, positive predictive value; NPV, negative predictive value.
Discussion
The present paper describes a pilot study comparing the perfusion assessment and quantification software, applying the SPY-Elite® and the SPY-PHI QP simultaneously (Video 4). The main aim was to investigate if the two software systems can be applied and perfusion values interpreted interchangeably. Secondly, to compare the per-operative quantitative perfusion assessment with postoperative outcomes 4-week after surgery.
When looking at the overall accessibility of the SPY-Elite® and the SPY-PHI QP, both systems are user friendly using near-infrared laser and intravenous administration of ICG to obtain the per-operative angiographies.
The SPY-PHI QP is the newest addition in the line of imaging systems, and has several features. The camera is lightweight, handheld and equipped with relevant control buttons enabling the surgeon to use the camera without leaving the surgical field of interest. The hand-held camera makes the angiography easy to conduct and the user can easily move the camera around (Figure 5).

According to the manufacturing company (Stryker AB, Malmö, Sweden, www.stryker.com), the relative values (% tissue perfusion) ought to be independent of the contour of the tissue. Though, in our experience, the relative percentages changed if the camera was not held horizontally to the surface.
In contrast to the SPY-Elite® system, the SPY-PHI QP can be applied independently of the lighting in the operating room, limiting possible interferences with the surgical team.
The SPY-PHI QP-system contains different visualization modes, however only the contrast mode/fluorescence mode was applied in this study.
The SPY-Elite® camera is attached to an arm located on the machine (Figure 6). Therefore, the recordings are more inflexible and rigid, though we found the results to be steadier and more comparable when compared to the hand-held recordings of the SPY-PHI QP.
The SPY-Elite® system has a built-in function with laser dots indicating the optimal distance from the tissue of interest. Whereas the SPY-PHI QP is held 10–40 cm from the tissue to obtain optimal recording, difficult to apply in a clinical setting (16,17).
The flexible distance interval of 10–40 cm results in a larger field of vision when using the SPY-PHI QP (Figure 7).
To obtain the quantification of the relative tissue perfusion, the user has to set a reference value (100% tissue perfusion) on e.g., the sternum/thorax or adjacent healthy tissue. When setting the 100% reference using the SPY-PHI QP, the camera needs to be relocated to the reference tissue outside the surgical field which changes the visual field.
After obtaining the 100% reference point, the camera is moved back to the surgical site which can interrupt assessment of perfusion assessment.
Overall, in our experience, the SPY-Elite® is superior in availability, ease of use and clinical applicable assessment of tissue perfusion.
Several authors have investigated cut-off values using the SPY-Q software (12-15). In implant-based breast reconstructions, Diep et al. applied a relative tissue perfusion cut-off <20% and Mattison et al. used contour values (10%, 15% and 20%) as cut-off values (29,30). Two studies reported a relative tissue perfusion <33% as threshold for tissue excision (15,31).
In autologous reconstruction one study by Wu et al. reported a cut-off value of 25% (32), while Alstrup et al. used 33% (18). Use of absolute perfusion values instead of relative perfusion have also been reported (12,13,33).
Since there exists no international consensus on specific cut-off values, we applied the relative perfusion cut-off value of 33% as reported by Moyer et al. (15,22-24).
Table 2 shows the quantitative measurements for each patient using both SPY-systems. Measurements were not equal in 4/16 cases (25%), but in 12/16 (75%) cases both systems quantified the perfusion above the cut-off value of >33%.
This finding could indicate that though the quantitative measurements of the two imaging systems may not be equated using a 33% cut-off, both systems are clinically applicable in assessing the per-operative perfusion.
Tables 3,4 shows the calculated sensitivity, specificity, PPV and NPV of the SPY-PHI QP and the SPY-Elite® system, respectively.
Overall, the results show that the two quantification software systems are not comparable when using a cut-off value at 33%. The SPY-PHI QP-system yielded a sensitivity of 50% with a PPV of 25%, indicating a low ability to identify cases with perfusion <33% and a corresponding low probability of finding actual cases with hypoperfusion. There was a high specificity of 77% with NPV of 91% indicating high ability to identify cases with sufficient perfusion.
The SPY-Elite® system yielded an equal sensitivity of 50% but with a PPV of 100%, indicating that when the SPY-Elite® detects an area with perfusion <33%, there is a probability of actual hypoperfusion of 100%. The specificity was 100% with an NPV of 100%.
As per standard from the paper by Moyer et al., these results indicate that the SPY-Elite® quantification system equals more valid results that the SPY-PHI QP system, when using a cut-off value of 33% (15).
The SPY-PHI QP had an accuracy of 73%, with the corresponding accuracy for the SPY-Elite® system being 93%. These results indicate that the two quantification software systems cannot be equated, and that we do not know the correct cut-off value for the SPY-PHI QP yet.
The risk equating and interpreting the results of the two systems using a cut-off value of 33%, would result in a substantial risk of (I) not identifying the true cases with hypoperfusion, (II) overestimating areas with hypoperfusion when using the SPY-PHI QP—with a risk of removing viable tissue.
The findings of this observational study support the conclusion, that these 2 quantification systems are not to be used and interpreted equally, though further studies including a larger sample size is needed.
Limitations
This study was conducted as a pilot study on a relatively small number of cases including several different breast reconstructive procedures with different effects on the vascularity. The small sample size and heterogeneity of the cases may limit the conclusions to be drawn from this study and therefore conclusions are suggestive rather than conclusive.
The complications in Case no. 2, 10 and 12 (postoperative hematoma and pulmonary embolism) occurred postoperatively and could possibly have led to decreased perfusion and complications, independently of the per-operative ICG-A.
This study is limited due to the observational and comparative design, and especially due to the small number of patients making the need for further studies with a greater sample size even more essential.
This is the first study to describe a head-to-head comparison of the two existing imaging devices (SPY-Elite imaging system® and the SPY-PHI QP system) able quantify relative tissue perfusion based on ICG-A applied on breast reconstructive surgery.
Conclusions
Using ICG-A for perfusion assessment and quantification is becoming increasing more popular in reconstructive surgery. A new edition to the imaging software systems is the SPY-PHI QP quantification software system.
This paper describes an observational pilot study comparing the SPY-Elite® with the SPY-PHI QP comparing perfusion assessment and quantitative perfusion values compared to postoperative complications.
The two imaging systems may not be applicable using the same cut-off value. Therefore, further studies investigating specific cut-off values for the SPY-QP is needed.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-123/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-123/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-123/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This is an observational study of two imaging modalities already approved for clinical use. There has not been any intervention. Therefore, ethical approval was not required. All data was obtained and stored anonymously. Inform consent was not required. This observational study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eren S, Rübben A, Krein R, et al. Assessment of microcirculation of an axial skin flap using indocyanine green fluorescence angiography. Plast Reconstr Surg 1995;96:1636-49. [Crossref] [PubMed]
- Pestana IA, Coan B, Erdmann D, et al. Early experience with fluorescent angiography in free-tissue transfer reconstruction. Plast Reconstr Surg 2009;123:1239-44. [Crossref] [PubMed]
- Holm C, Mayr M, Höfter E, et al. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg 2002;55:635-44. [Crossref] [PubMed]
- Zenn MR. Fluorescent angiography. Clin Plast Surg 2011;38:293-300. [Crossref] [PubMed]
- Flower RW, Hochheimer BF. A clinical technique and apparatus for simultaneous angiography of the separate retinal and choroidal circulations. Invest Ophthalmol 1973;12:248-61. [PubMed]
- Varela R, Casado-Sanchez C, Zarbakhsh S, et al. Outcomes of DIEP Flap and Fluorescent Angiography: A Randomized Controlled Clinical Trial. Plast Reconstr Surg 2020;145:1-10. [Crossref] [PubMed]
- Parmeshwar N, Sultan SM, Kim EA, et al. A Systematic Review of the Utility of Indocyanine Angiography in Autologous Breast Reconstruction. Ann Plast Surg 2021;86:601-6. [Crossref] [PubMed]
- Pruimboom T, Schols RM, Van Kuijk SM, et al. Indocyanine green angiography for preventing postoperative mastectomy skin flap necrosis in immediate breast reconstruction. Cochrane Database Syst Rev 2020;4:CD013280. [Crossref] [PubMed]
- Lauritzen E, Damsgaard TE. Use of Indocyanine Green Angiography decreases the risk of complications in autologous- and implant-based breast reconstruction: A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2021;74:1703-17. [Crossref] [PubMed]
- Karakawa R, Yano T, Shibata T, et al. Use of the wearable smart glasses for indocyanine green (ICG) angiography of a flap surgery. Microsurgery 2020;40:276-7. [Crossref] [PubMed]
- Damsgaard TE, Rønning H. Indocyanine green guided mastectomy and immediate breast reconstruction. Gland Surg 2019;8:S287-90. [Crossref] [PubMed]
- Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 2012;129:778e-88e. [Crossref] [PubMed]
- Munabi NC, Olorunnipa OB, Goltsman D, et al. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg 2014;67:449-55. [Crossref] [PubMed]
- Gurtner GC, Jones GE, Neligan PC, et al. Intraoperative laser angiography using the SPY system: review of the literature and recommendations for use. Ann Surg Innov Res 2013;7:1. [Crossref] [PubMed]
- Moyer HR, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plast Reconstr Surg 2012;129:1043-8. [Crossref] [PubMed]
- SPY-PHI SPY Portable Handheld Imaging System. April. 2021. Available online: https://www.stryker.com/us/en/endoscopy/products/spy-phi.html
- Novadaq Technologies. SPY-QP Fluorescence Assessment Software. (User Guide, International). Available online: https://www.stryker.com
- Alstrup T, Christensen BO, Damsgaard TE. ICG angiography in immediate and delayed autologous breast reconstructions: peroperative evaluation and postoperative outcomes. J Plast Surg Hand Surg 2018;52:307-11. [Crossref] [PubMed]
- Girard N, Delomenie M, Malhaire C, et al. Innovative DIEP flap perfusion evaluation tool: Qualitative and quantitative analysis of indocyanine green-based fluorescence angiography with the SPY-Q proprietary software. PLoS One 2019;14:e0217698. [Crossref] [PubMed]
- Griffiths M, Chae MP, Rozen WM. Indocyanine green-based fluorescent angiography in breast reconstruction. Gland Surg 2016;5:133-49. [PubMed]
- Burnier P, Niddam J, Bosc R, et al. Indocyanine green applications in plastic surgery: A review of the literature. J Plast Reconstr Aesthet Surg 2017;70:814-27. [Crossref] [PubMed]
- Johnson AC, Colakoglu S, Chong TW, et al. Indocyanine Green Angiography in Breast Reconstruction: Utility, Limitations, and Search for Standardization. Plast Reconstr Surg Glob Open 2020;8:e2694. [Crossref] [PubMed]
- Liu EH, Zhu SL, Hu J, et al. Intraoperative SPY Reduces Post-mastectomy Skin Flap Complications: A Systematic Review and Meta-Analysis. Plast Reconstr Surg Glob Open 2019;7:e2060. [Crossref] [PubMed]
- Driessen C, Arnardottir TH, Lorenzo AR, et al. How should indocyanine green dye angiography be assessed to best predict mastectomy skin flap necrosis? A systematic review. J Plast Reconstr Aesthet Surg 2020;73:1031-42. [Crossref] [PubMed]
- Malagón-López P, Vilà J, Carrasco-López C, et al. Intraoperative Indocyanine Green Angiography for Fat Necrosis Reduction in the Deep Inferior Epigastric Perforator (DIEP) Flap. Aesthet Surg J 2019;39:NP45-54. [Crossref] [PubMed]
- Hembd AS, Yan J, Zhu H, et al. Intraoperative Assessment of DIEP Flap Breast Reconstruction Using Indocyanine Green Angiography: Reduction of Fat Necrosis, Resection Volumes, and Postoperative Surveillance. Plast Reconstr Surg 2020;146:1e-10e. [Crossref] [PubMed]
- Hembd A, Teotia SS, Zhu H, et al. Optimizing Perforator Selection: A Multivariable Analysis of Predictors for Fat Necrosis and Abdominal Morbidity in DIEP Flap Breast Reconstruction. Plast Reconstr Surg 2018;142:583-92. [Crossref] [PubMed]
- Momeni A, Sheckter C. Intraoperative Laser-Assisted Indocyanine Green Imaging Can Reduce the Rate of Fat Necrosis in Microsurgical Breast Reconstruction. Plast Reconstr Surg 2020;145:507e-13e. [Crossref] [PubMed]
- Diep GK, Marmor S, Kizy S, et al. The use of indocyanine green angiography in postmastectomy reconstruction: Do outcomes improve over time? J Plast Reconstr Aesthet Surg 2019;72:548-54. [Crossref] [PubMed]
- Mattison GL, Lewis PG, Gupta SC, et al. SPY Imaging Use in Postmastectomy Breast Reconstruction Patients: Preventative or Overly Conservative? Plast Reconstr Surg 2016;138:15e-21e. [Crossref] [PubMed]
- Hammer-Hansen N, Juhl AA, Damsgaard TE. Laser-assisted indocyanine green angiography in implant-based immediate breast reconstruction: a retrospective study. J Plast Surg Hand Surg 2018;52:158-62. [Crossref] [PubMed]
- Wu C, Kim S, Halvorson EG. Laser-assisted indocyanine green angiography: a critical appraisal. Ann Plast Surg 2013;70:613-9. [Crossref] [PubMed]
- Mirhaidari SJ, Beddell GM, Orlando MV, et al. A Prospective Study of Immediate Breast Reconstruction with Laser-Assisted Indocyanine Green Angiography. Plast Reconstr Surg Glob Open 2018;6:e1774. [Crossref] [PubMed]
Cite this article as: Lauritzen E, Bredgaard R, Bonde C, Jensen LT, Engberg Damsgaard T. An observational study comparing the SPY-Elite®vs. the SPY-PHI QP system in breast reconstructive surgery. Ann Breast Surg 2023;7:12.