
Transaxillary endoscopic breast reconstruction: case series from single institution
Introduction
Breast cancer (BRCA) is the most commonly diagnosed cancer in women (1). The estimated incidence amongst European countries has been 576,300 in 2020 and 21% of BRCA cases in Europe occur in women when they are younger than 50 years old (1,2). A higher frequency of early onset female BRCAs has been observed in the last decades. Younger patients diagnosed with BRCA are more likely to ask for breast reconstruction and expect good to excellent cosmetic outcomes. For such a purpose, minimally invasive surgical techniques have been developed and improved to yield the best cosmetic results without jeopardizing the oncological safety (3,4).
The gold standard for early BRCA treatment is breast conserving plus axillary surgery. However, for multicentric tumours, large breast tumours and BRCA gene carrier subcutaneous mastectomy is mainly performed (5). Subcutaneous mastectomies can be performed along with direct to implant (DTI) reconstruction or as a two-stage surgery by positioning a tissue expander under the muscular pocket. Tissue expansion varies from 3 to 6 months and second stage surgery is generally performed at least 6 to 8 months after (6). Mastectomy incision may be variable. It can be periareolar, lateral or inframammary (7). The periareolar incision results in a higher rate of nipple necrosis. Using the lateral or inframammary incision reduces the incidence of nipple necrosis and may help improve overall reconstructive and cosmetic outcomes (7). Despite this, some patients willing to have less visible scars and minimally invasive surgical techniques are often requested. Endoscopic-assisted breast surgery represents a minimally invasive technique that allows surgeon to work within a small incision through a rounded instrument called endoscope (8). Several single institutions have been shown results with endoscopic-assisted breast surgery (9-11). First described for breast augmentation, endoscopic assisted breast surgery (EABS) result in great patient satisfaction without critical complications (8). Nowadays the use of EABS is gaining ground to treat BRCA although literature is still scant (12,13). EABS can be used for subcutaneous mastectomy along with DTI reconstruction as well as for two-stage breast surgery (14,15). Here we report our single institution experience with EABS amongst a subset of patients who were scheduled for two-stage surgery in order to evaluate the feasibility of this technique. We present the following article in accordance with the STROBE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-143/rc).
Methods
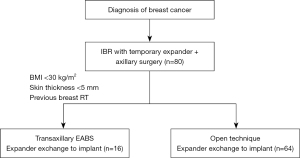
Here we report a retrospective analysis from our Institution. Between 2020 and 2021, we performed eighty breast reconstruction procedures at National Cancer Institute of Naples. Sixteen out of them received diagnosed with BRCA and underwent mastectomy and reconstruction with tissue expander received the second stage surgery with endoscopic video assisted single-port technique. Inclusion criteria for EABS technique was diagnosis of BRCA; previous breast reconstruction with tissue expander; BMI <30 kg/m2; previous breast irradiation; skin flap thickness <5 mm, as shown in the flow chart in Figure 1. There were no limits of age. Smoke was not an exclusion criterion. During surgery tissue expander was removed and replaced with prosthetic implant through axillary scar with endoscopic-assisted technique. Informed consent was discussed and signed before surgery by all the patients. Indications for EABS were previous nipple sparing mastectomy for BRCA.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was not required by Ethics Committee since this is a retrospective case series analyses according to SAGE guidelines. All patients gave informed consent before having their surgery.
Statistical analysis
Statistical analyses were performed using SPSS version 26. Differences in demographic and clinical variables were analyzed using independent sample t-tests for age. Sample size was less than 30, thus we have been used the population standard deviation with confidence interval (CI) at 95%.
Surgical procedure
Patients were operated under general anesthesia and before surgery had ultrasound guided erector spinae plane (ESP) block and pectoralis (PECS) block (16,17). This block provides analgesia by targeting the dorsal and ventral branches of the spinal nerves. The ESP block was performed by depositing the local anesthetic in the fascial plane, deeper than the erector spinae muscle at the tip of the transverse process of the vertebra. The PECS block provides analgesia by targeting the interfacial planes between the PECS major and the PECS minor muscles at the fourth rib level. The PECS II injection occurs between the serratus anterior muscle and PECS minor muscle at the same rib level. The technique was guided with a high-frequency linear ultrasound transducer by the anesthesiologist. The probe was placed in a transverse orientation to identify the spinous process. Once the level was identified, the probe was moved 3 cm laterally until the transverse process was identified. Three muscles must be identified the trapezius, rhomboid major, and erector spinae. The patient was then positioned supine on the table in the operating room. Arms were abducted to 90 degrees in order to expose the axilla and to avoid brachial plexus injuries.
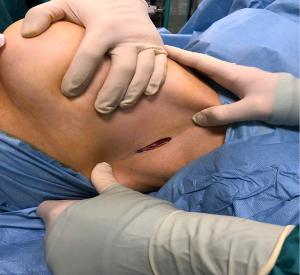
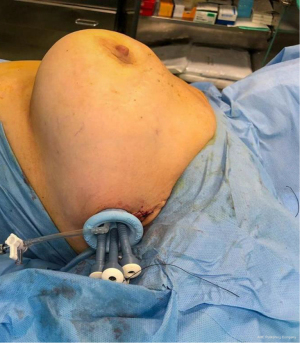
Firstly, 3 to 5 cm long incision was performed under the axillary fold (Figure 2, Figure 3A,3B). All incisions were done by cutting through existing scar tissue from previous surgery either sentinel node biopsy or complete axillary dissection. After that subcutaneous flap was opened the tissue expander was detached from the muscular pocket and removed from axillary scar. Then an endoscopic instrument called Single-Incision Laparoscopic Surgery port (SILSTM port-Medtronic Limited, Watford WD 18, UK) with two 5-mm trocars and one 10-mm trocar was inserted into the incision (Figure 4). Pneumo-breast was established at a pressure of 8–10 mmHg in order to create a working space. A 10-mm 30° camera was used. Five mm atraumatic grasper, laparoscopic aspirator and laparoscopic hook for dissection or Ultracision (Johnson & Johnsons, Zug, Switzerland) were used. Thereafter capsulectomy was performed. Small bleeding was cauterized and the pocket was irrigated with saline. At this point the single-port the surgeon was removed and breast implant was inserted within the pocket. Implants were all smooth to better slip into the muscular pocket within the small scar. When necessary, a matrix was applied to cover the lower-lateral pole when the pocket appeared too tight. It was stitched laterally to the PECS major muscle and down to the inframammary fold, with the breast prosthesis placed underneath. Nineteen-Fr suction drains were placed in submuscular plane and the pocket was closed laterally. The subcutaneous layer was closed with 4-0 Vicryl Rapid Undyed (Ethicon, Somerville, NJ, USA). The skin was closed using 5-0 absorbable suture.
Technologies and device
The significant advancements in medical technology in recent years, with the development of new instruments and new technologies, has thrived a rapid growth of several types of minimally invasive surgery, like EABS, providing substantial benefits over the traditional technique. The principal devices utilized in the surgical procedure are SILS port, Ultracision scalpel or monopolar laparoscopic hook, High definition 30° camera, the conventional laparoscopic instruments and the set-up, which are already available in any unit performing minimally invasive surgery.
The SILS port is a common device generally used in abdominal and transanal minimally invasive surgery with several advantages. In the case of EABS, its smaller diameter (30 mm) guarantees small and then lower visible incisions, and its pliability reduces the ischemia and traumatism of the skin in contrast to the rigid retractors used in traditional open surgery. The high-resolution images, the good illumination of an endoscopic view in an otherwise dark environment, and the possibility that offer the vision of 30° camera, it is possible to control, easily and safely, the common sources of bleeding generally arising from muscles and soft tissue. Transillumination helps surgeon to visualize skin vascularization (Figure 5).
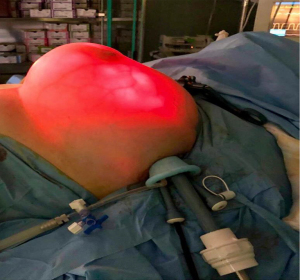
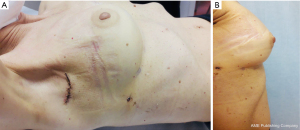
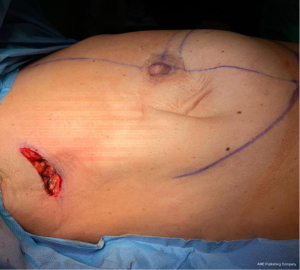
EABS allows better visualization of the internal surgical field with a contemporary direct external vision instead of viewing of the traditional surgery. In the case of EABS two-stage breast reconstruction, the surgeon may modulate the inside periprosthetic capsule dissection with direct visualization of the skin, redefining with precision the breast boundary and therefore building a tailored prosthetic pocket. The pneumo-breast, stretching out the new prosthetic pocket, allows to measuring the actual volume and lead the dissection until the surgeon does not achieve the tailored volume and shape. Transillumination is used to identify subcutaneous vascular texture to avoid the injury of the blood supply of the nipple. Dissection was done generally with monopolar hook or Ultracision, tools that convert electrical energy into mechanical motion, effectively cutting through tissue via high-frequency vibrations of his blade. The scar is hidden in the axillary crease (Figure 6).
Results
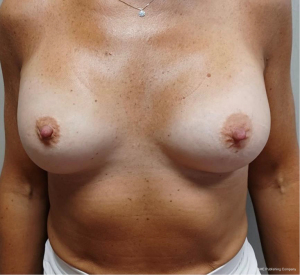
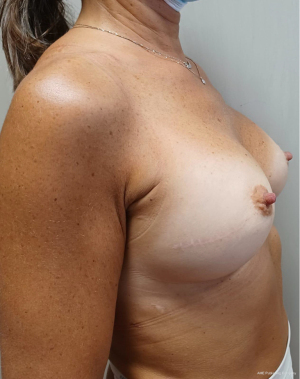
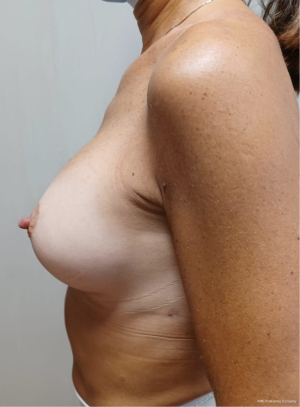
Sixteen patients underwent tissue expander removal and prosthetic implant replacement with single-port EABS. Only sixteen out of eighty were selected for transaxillary procedure because at risk of major complications after radiation therapy and skin thickening. Patients mean age was 56 years (range, 45–66 years). The learning curve was fast overall with mean operative time of 134 minutes varying from 60 to 240 minutes. Mean implant volume of permanent implants was 440 cc (265–630 cc) (Table 1). Six out of 16 patients were cigarette smokers. Five out 16 had contralateral breast surgery at the same time. One out of this five was a BRCA1 carrier and a contralateral risk-reducing mastectomy. Four out of 5 patients had contralateral symmetrisation (one augmentation and three wise pattern reduction mammaplasty) (Figures 7-10). One patient underwent nipple reconstruction. Fifty percent of patients (8 out of 16) underwent irradiation after immediate reconstruction tissue expander because of the presence of metastatic lymph nodes at final pathology report at the time of first operation. Median follow-up after EABS is eight months (6–12 months). All procedures were performed without intraoperative and post-operative complications. None of the patients developed “need to treat” seroma, haematoma, infection or skin dehiscence. Patients were follow-up weekly during the first month after surgery and monthly for the first semester. Then were scheduled for clinical examination every 6 months in the Outpatient Plastic and Reconstructive Surgery Clinic and annual magnetic resonance imaging (MRI).
Table 1
| Variables | Value | Range | 95% CI |
|---|---|---|---|
| Mean age (years) | 56 | 45–66 | 53.5–58.5 |
| Mean implant volume (cc) | 440 | 265–630 | 438–442 |
| Mean BMI (kg/m2) | 25 | 20–29 | 22.6–27.4 |
| Mean operation time (min) | 134 | 60–240 | 132–136 |
BMI, body mass index; CI, confidence interval.

Discussion
EABS is a minimally invasive technique that allows surgeons to perform endoscopically either nipple sparing mastectomies along with axillary surgery or breast expander exchange amongst high-risk cases after skin irradiation (15,18). EABS allows a significant reduction of trauma to the patient’s body that results from the minimization of surgical incisions. The reduced physical trauma leads to several additional benefits for the patient: minimal cosmetic disfiguration, reduced pain, lower incidence of post-surgery complications, quicker recovery, shorter length of hospital stay, decreased psychological impact and overall improved quality of life. The primary advantage of the transaxillary breast procedures is that the incision is hidden from view, thus it is strongly accepted by patients. The second big advantage of transaxillary approach is sparing irradiated or attenuated skin from stretching and stitching avoiding either to cut or to “re-cut” the previously irradiated breast skin. The benefit of not re-opening the previous breast incision in the second stage breast reconstruction surgery is the reduction of implant exposure and skin dehiscence. Moreover, the transaxillary technique allowed the surgeon to choose amongst larger implant volumes without impact on the skin wound. One of the disadvantages of transaxillary procedures is that plastic surgeons often have less control overall position of the breast implant when working through the armpit. This could mean some difficulty when it comes to placing the breast implant under the pectoral muscle. However, this drawback can be easily overcome by carefully selecting patients and this approach can be discussed more with the patient before scheduling surgery. Secondly implants used should be preferably smooth because they can easily slip into the pocket through the small axillary incision. Lastly, costs represent a big disadvantage of EABS since expensive devices are involved.
Conclusions
EABS after mastectomy has not yet become a standard procedure for BRCA reconstruction, although several case series have been reported in the current literature with good or excellent results. The use of expensive devices may affect the spreading of this technique totally accepted by patients. In our own experience, EABS has given good results in a small subset of patients scheduled for a second stage breast surgery willing to avoid a breast scar or amongst woman who had breast irradiation more likely to develop local complications like skin dehiscence or implant exposure.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Nicola Rocco) for the series “New Perspectives in Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-143/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-143/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-143/coif). The series “New Perspectives in Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was not required by Ethics Committee since this is a retrospective case series analyses according to SAGE guidelines. All patients gave informed consent before having their surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. Int J Cancer 2021; [Crossref] [PubMed]
-
EU-27 Bcbi - Eaves FF 3rd, Bostwick J 3rd, Nahai F, et al. Endoscopic techniques in aesthetic breast surgery. Augmentation, mastectomy, biopsy, capsulotomy, capsulorrhaphy, reduction, mastopexy, and reconstructive techniques. Clin Plast Surg 1995;22:683-95. [Crossref] [PubMed]
- Tsangaris TN, Trad K, Brody FJ, et al. Endoscopic axillary exploration and sentinel lymphadenectomy. Surg Endosc 1999;13:43-7. [Crossref] [PubMed]
- Petit JY, Veronesi U, Luini A, et al. When mastectomy becomes inevitable: the nipple-sparing approach. Breast 2005;14:527-31. [Crossref] [PubMed]
- Bellini E, Pesce M, Santi P, et al. Two-Stage Tissue-Expander Breast Reconstruction: A Focus on the Surgical Technique. Biomed Res Int 2017;2017:1791546. [Crossref] [PubMed]
- Rawlani V, Fiuk J, Johnson SA, et al. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. Can J Plast Surg 2011;19:129-33. [Crossref] [PubMed]
- Momeni A, Padron NT, Bannasch H, et al. Endoscopic transaxillary subpectoral augmentation mammaplasty: a safe and predictable procedure. J Plast Reconstr Aesthet Surg 2006;59:1076-81. [Crossref] [PubMed]
- Lai HW, Chen ST, Chen DR, et al. Current Trends in and Indications for Endoscopy-Assisted Breast Surgery for Breast Cancer: Results from a Six-Year Study Conducted by the Taiwan Endoscopic Breast Surgery Cooperative Group. PLoS One 2016;11:e0150310. [Crossref] [PubMed]
- Franceschini G, Visconti G, Garganese G, et al. Therapeutic nipple-sparing mastectomy combined with endoscopic immediate prosthetic breast reconstruction via axillary incision. A further step towards evidence-based and personalized surgery. Ann Ital Chir 2020;91:417-25. [PubMed]
- Peralta-Castillo GG, Cavazos-García R, Eulalia-Hernández E, et al. Single port endoscopic mastectomy: surgical technique and first case in Mexico. Cir Cir 2020;88:108-12. [PubMed]
- Visconti G, Franceschini G, Barone-Adesi L, et al. Transaxillary Nipple-Sparing Mastectomy, Lymphadenectomy and Direct-to-Implant Submuscular Breast Reconstruction Using Endoscopic Technique: A Step toward the "Aesthetic Mastectomy". Plast Reconstr Surg 2019;143:1122e-3e. [Crossref] [PubMed]
- Lai HW, Chen ST, Mok CW, et al. Single-port 3-dimensional Videoscope-assisted Endoscopic Nipple-sparing Mastectomy in the Management of Breast Cancer. Plast Reconstr Surg Glob Open 2019;7:e2367. [Crossref] [PubMed]
- Tukenmez M, Ozden BC, Agcaoglu O, et al. Videoendoscopic single-port nipple-sparing mastectomy and immediate reconstruction. J Laparoendosc Adv Surg Tech A 2014;24:77-82. [Crossref] [PubMed]
- Eberlin KR, Gfrerer L, Liao EC. Trans-axillary approach for breast implant exchange in high risk cases of irradiated or attenuated skin. J Plast Reconstr Aesthet Surg 2014;67:1624-9. [Crossref] [PubMed]
- Kot P, Rodriguez P, Granell M, et al. The erector spinae plane block: a narrative review. Korean J Anesthesiol 2019;72:209-20. [Crossref] [PubMed]
- Woodworth GE, Ivie RMJ, Nelson SM, et al. Perioperative Breast Analgesia: A Qualitative Review of Anatomy and Regional Techniques. Reg Anesth Pain Med 2017;42:609-31. [Crossref] [PubMed]
- Lai HW, Toesca A, Sarfati B, et al. Consensus Statement on Robotic Mastectomy-Expert Panel From International Endoscopic and Robotic Breast Surgery Symposium (IERBS) 2019. Ann Surg 2020;271:1005-12. [Crossref] [PubMed]
Cite this article as: Esposito E, Marone U, Saponara R, Morra E, Di Monta G, Rho M, Avino F, Mori S. Transaxillary endoscopic breast reconstruction: case series from single institution. Ann Breast Surg 2023;7:25.

