Current concepts of lymphedema treatment for the breast cancer patient: a clinical practice review
Lymphedema is a progressive disease of the lymphatic system characterized by accumulation of proteins in the interstitium leading to chronic inflammation, adipose deposition and ultimately fibrosis of the skin and subcutaneous tissues. The most common cause of secondary lymphedema in the developed world is cancer treatment with breast malignancy in the lead. It is estimated that as many as 50% of breast cancer patients treated with axillary lymph node dissection go on to develop lymphedema (1,2). In the case of the less invasive sentinel lymph node biopsy (SLNB), it is estimated to be around 5% (3). This rate of lymphedema may be secondary to disruption of arm lymphatics during an SLNB procedure. Identifying and preserving the arm nodes with reverse axillary mapping may translate into a lower incidence of lymphedema with SLNB and axillary lymph node dissection (4). Lymphedema may arise at any time, months or even years after breast cancer surgery, but approximately 75% of cases occur in the first year after surgery (5,6). The treatment for lymphedema in breast cancer patients should be multi-disciplinary and targeted on balancing this chronic illness. Patients should be informed nowadays that there are vast treatment options for treating lymphedema.
Clinical features and evaluation
Lymphedema is a progressive process with worsening symptoms. At first, patients describe a sense of discomfort and heaviness. This may later proceed by chronic swelling, pitting edema and recurrent cellulitis. With time, patients may go on to develop non-pitting edema and eventually elephantiasis. This shift, from pitting to non-pitting edema, represents hypertrophy and fat deposition of the interstitium which, at the end stage, can result in overt fibrosis.
There are numerous classification systems for grading lymphedema. Some like The International Society of Lymphology staging system (ISL) rely primarily on clinical features of the disease (7). Other classifications are aided on imaging based on indocyanine green (ICG) findings (8,9). The ISL system is the most widely used and portray clinical findings of the limb (Table 1, Figure 1). Patients with stages 1 and 2 lymphedema may benefit from a microsurgical reconstruction.
Table 1
| Stage 0: subclinical condition in which swelling is not evident despite impaired lymph transport |
| Stage 1: early reversible pitting edema; limb elevation will reduce swelling |
| Stage 2: irreversible lymphedema; pitting is no longer present |
| Stage 3: end-stage lymphedema with elephantiasis; pitting is absent and trophic skin changes (acanthosis, fat deposits, and wart overgrowths) develop |
The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology (updated 2020).
Those with a graver stage (2 and 3), characterized by fat hypertrophy and fibrosis, were once limited to debulking surgery alone but today, with evolving knowledge of the disease and treatment options, the surgical treatment is tailored to the patient and different surgical modalities are proposed and combined in the same surgery.
Pre-op assessment of a swollen limb should consider other possible pathologies and risk factors. Obesity is a great contributor of secondary lymphedema. Studies have shown an almost linear correlation with higher baseline weight and the development of lymphedema after axillary lymph node dissection for breast cancer (2). For this reason, obesity must be addressed with weight loss ahead of surgical treatment whenever possible (10). Other risk factors for the development of secondary lymphedema include axillary lymph node dissection or dissection of four or more nodes. Radiotherapy alone, and to a greater extent when combined with ALND, is another major risk factor.
The diagnosis of lymphedema is clinical and high suspicion is sufficient. Imaging is a very useful tool to explore the extent of the disease, but it is not mandatory for the diagnosis. A thorough medical history should be recorded, emphasizing on the above-mentioned risk factors. Measurements of the limb are performed using optoelectronic limb volumeter (Perometer). This is a reliable and convenient tool for measuring limb volume with each measurement taking only a few seconds.
The psychosocial impact on the patient is often neglected. These women, some of whom are still very young, have been confronted with breast cancer and its deleterious effect on their well-being, self-esteem, and sexuality. On top of their oncological treatments, they suffer a major complication that warrants further treatments and more time away from home, friends, and work. This issue needs to be addressed with sensitivity and these patients should receive professional help from therapists and social workers. A multi-disciplinary, holistic approach should guide the course of treatment.
Conservative therapy
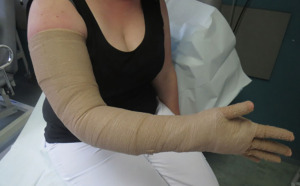
The standard nonsurgical therapy for lymphedema is complete decongestive physiotherapy (CDT). This multimodality approach combines the use of manual lymphatic drainage, bandaging, exercise, and skin care. CDT comprises two consecutive phases: Phase 1 is the initial reduction phase in which patients are subjected to five therapy sessions per week, in times combined with elastic bandaging (Figure 2). It usually takes 4 to 8 weeks for the reduction in fluid to reach a plateau. Phase 2 is the maintenance phase where the aim is to preserve the reduction obtained. At this stage, manual lymphatic drainage is less frequent (1–3 times/week) and compression garments are applied. This time consuming, lifelong commitment is aimed to reduce the progression of the disease with its complications. Although recent studies have raised doubts regarding its impact, it is still considered first-line therapy for lymphedema (11). All patients are requested for a minimum of 6-month trial of decongestive therapy prior to considering a possible surgical intervention, especially in the early stages of the disease.
Assessment and imaging
Diagnostic imagining techniques are essential tools to determine the scale of disease progression in order to tailor an appropriate therapeutic strategy for each patient. It can also be helpful for assessing post-operative improvement. The three main imaging modalities for lymphedema patients are radionuclide lymphoscintigraphy (LSG), near infra-red (NIR) fluorescence, and magnetic resonance lymphangiography (MRL).
Radionuclide lymphoscintigraphy (LSG)
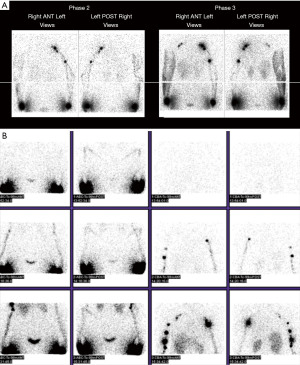
Lymphoscintigraphy is an imaging test that gives a global evaluation of the functionality of the lymphatic system and is the reference standard for confirming the diagnosis of lymphedema. It uses technetium-labeled colloid and nuclear scanning through a recommended protocol for LSG. The colloid is injected sub-dermally in one or more web spaces of the hand. A minimum of 3 images are obtained: 30 minutes after injection, another after 15 minutes of finger exercises, and one 60 minutes after normal activity. The radiologic images are then assessed to include: (I) the course of the radioactive tracer from the injection site to the axilla, (II) the transition time to the axilla, (III) the absence or presence of major lymphatic basins, (IV) the number and size of vessels and nodes, (V) the presence of collaterals, (VI) reflux, and (VII) relative symmetry compared with the opposite limb. The transport index represents the uptake of the colloid and how quickly it reaches the nodal basin. Leakage out of damaged lymphatic vessels is reflected by reflux of the dye (12). Typical images of lymphoscintigraphy can be seen in Figure 3A,3B. The ability to assess flow velocity of the lymphatic system, quantification and nodal basin is the main advantage of LSG. It can also provide comparative information for postoperative assessment. Its downfalls include poor anatomic resolution for it does not provide any anatomic information in 3D. It is unable to assess interstitial tissues nor give indication of fat hypertrophy and it is a lengthy procedure. For this reason, information must often be complemented with ICG-lymphography and MR-lymphangiography.
Near infra-red (NIR) fluorescence imaging—indocyanine green (ICG) lymphography
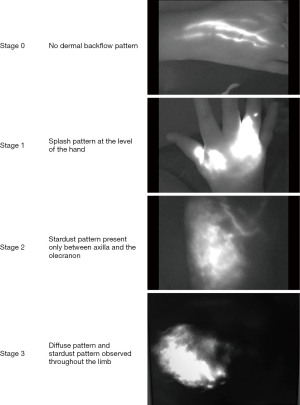
NIR fluorescence visualizes real-time function of the superficial lymphatic vessels. The tracer, ICG, is injected subcutaneously at the web spaces of the hand followed by a near-infrared camera scan. The images can identify the location and functional status of superficial lymphatic vessels. In a healthy limb, flow through lymphatic vessels is detected spontaneously, even with minimal mobilization. In a failing lymphatic system however, the dye migration may be slowed down or fully impaired, might necessitate manual drainage or extravasate through the lymphatic vessel walls. In patients with diseased lymphatics, the number of functional vessels is reduced, and reflux (dermal backflow) might be seen. These findings were gathered to form a staging system developed by Yamamoto et al. where normally functioning lymphatic vessels appear as linear structures (13). With disease progression, the pattern visualized will shift from a linear pattern through a splash pattern, a stardust pattern and finally a diffuse pattern in advanced cases (Figure 4). The procedure is limited by its ability to only visualize lymphatics that are less than 12 mm deep to skin surface and the field can be totally obliterated by the tracer, making the mapping difficult. It does not provide information regarding the interstitial tissues such as deep edema accumulation or fat hypertrophy and does not visualize venous patterns (14). For this reason, MR-lymphangiography, is advocated in selected cases.
Magnetic resonance lymphangiography (MRL)
MRL combines magnetic resonance imaging technology with a lymphatic dye to allow precise anatomic imaging. It shows both the superficial and deeper lymphatic structures and provides high-quality images of the lymphatics, venules, and subcutaneous tissues. Mapping lymphatic vessels in relation to veins is difficult with standard imaging techniques. MRL has been shown to be accurate and sensitive compared in detecting anatomical abnormalities in the lymphatic system of patients with extremity lymphedema (15). Gadolinium is injected sub-dermally to all web spaces and is preferentially taken up by the lymphatics. The information gained includes the functionality of the lymphatic channels and visualization of the lymph node basin. The interstitial tissue along with presence and location of the edema is also demonstrated (16). This aids in disease staging and surgical planning. The combination of the functionality, location and quality of the lymphatic channels and its comparison to the interstitial tissues will determine the choice of surgical technique. With worsening lymphedema, the interstitial tissues will progress from fluid dominant edema to adipose dominant and finally to fibrosclerotic dominant. Microsurgical reconstruction remains an option for patients with fluid dominant edema with a relatively healthy subcutaneous tissue. Liposuction is used for those with adipose dominant limb swelling. Identifying the location and quality of the lymphatic vessels and venules helps to select the most suitable lymphatic channels for creating shunts during lympho-venous anastomosis. It has been demonstrated that MR lymphangiography is a good and accurate technique for pre-operative mapping of functional lymphatics and adjacent veins in the lymphedematous limb, thus improving patient selection for a feasible LVA (17). The examination gives us important anatomic information based on one imaging modality, that other examinations fail to give in such extent. In comparison with CT mapping of perforator flaps [e.g., deep inferior epigastric perforator (DIEP) flap], it makes surgery more predictable and swifter by giving exact coordinates for the place of incision in LVA (18). The downfalls of MRL are that it is time-consuming, requires a high level of expertise and is relatively expensive. On the other hand, information gathered from the MRL is that of both the LSC and the NIR fluorescence imaging, thus it might even be more cost effective.
Advantages and disadvantages of the different imaging techniques are listed in Table 2.
Table 2
| Lymphoscintigraphy | Infra-red fluorescence (ICG) | MR lymphography | |
|---|---|---|---|
| Lymphatic channels | + | + | + |
| 3D localization | − | +/− | + |
| Lymph nodes | + | − | + |
| Dermal backflow | + | + | + |
| Velocity progression | + | +/− | +/− |
| Fat deposition/fibrosis | − | − | + |
| Info veins | − | − | + |
| Info depth of lymphatic channels | − | Maximum 10–12 mm (sub-dermal) | Deeper lymphatic channels seen, and exact depth can be measured |
ICG, indocyanine green; MR, magnetic resonance.
Selection of treatment
Surgical management
Lymphedema treatment is aimed to restore lymphatic function and reduce adipose hypertrophy when installed. Surgical treatment for lymphedema is divided into reductive or physiologic techniques. Reductive techniques include resection or liposuction, and physiologic techniques involve reconstructive microsurgery.
Physiologic treatment
Microsurgical reconstructive techniques
Lymphatico-venous anastomosis (LVA)
Lymphatic fluid in the lymph vessels drains into the venous system via the thoracic ducts. LVA involves suturing superficial lymphatics to subdermal venules, creating peripheral shunts within the diseased limb, allowing a draining gradient between the congested high pressure lymphatic system and the lower-pressure venous system. Intact and functional lymphatic vessels are key components in LVA, and these can be confirmed with ICG prior to surgery. MRL can map the optimal locations to perform LVA where healthy lymphatics and venules are in proximity. These are also located during surgery using ICG and patent blue. Using this GPS mapping created by pre-operative MRL, two to four short incisions (2–3 cm long) are made just over the sites of healthy lymphatics, and when an adjacent venule is located, the anastomosis is performed using super-microsurgery techniques. The most used is an end-to-end anastomosis but other techniques have been described (19). The patency of the anastomosis can be tested with ICG or patent blue or by performing the ‘milking test’. The ideal number of anastomoses is yet to be determined; however, it is recommended to have as many as possible. As described, healthy functioning superficial lymphatics are essential for LVA, thus suitable candidates have stage 0 or 1 with minimal irreversible tissue fibrosis. At later stages LVA can sometimes still be performed but will not reverse fat hypertrophy or hard fibrosis. Studies have demonstrated volumetric improvements and symptoms relief including decreased incidence of cellulitis (9). Some patients were even able to completely discontinue use of compression garments, even over long-term follow-up (20). Some groups have embraced a prophylactic surgery that involves the anastomosis of arm lymphatics with a collateral branch of the axillary vein at the time of nodal dissection for the prevention of lymphedema (21). Lymphatic microsurgical preventing healing approach (LYMPHA) involves the injection of blue dye into the upper arm to map and preserve the arm lymphatic drainage during ALND and thus diminish lymphedema. Lymphatics coming from the arm (usually 2 to 4) are visible and by using multiple LVA’s between them and a secondary branch of the axillary vein, prevention of lymphedema is feasible. It is better considered in high BMI patients, those with 4 or more positive lymph nodes and those who require combined radiotherapy and LND. Boccardo et al. reported that only 3 out of 74 patients undergoing this procedure developed lymphedema. This 4% risk is much more favorable in comparison to up to 50% incidence of lymphedema in women undergoing ALND (22). Despite these results further study is needed to determine its efficacy on the long term.
Vascularized lymph node transfer (VLNT)
VLNT replenishes the missing lymph nodes by delivering vascularized tissue-containing lymph nodes from one area of the body to the affected limb as a free tissue transfer. For the upper limb lymphedema, the groin is the most popular donor site where the superficial inguinal lymph nodes are harvested based on either the superficial circumflex iliac or the superficial inferior epigastric vessels. Logically a lymph node flap is transferred anatomically to the axilla (where the nodes have been removed) although nodes can be placed extra-anatomically (elbow or wrist). The theory behind placement in a non-anatomic site is that the relocated lymph nodes act as a pump directing interstitial fluid into the venous network. This concept is also supported by post VLNT radionucleotide lymphoscintigraphy that visualizes the rerouted lymph fluid flowing into the recipient vein via the free flap nodes (23). Distal recipient sites may be more efficacious (24) and they allow gravitational drainage of the limb through the potential opening of old lymphatic channels once interstitial pressures normalize. As mentioned, upper extremity lymphedema is usually the result of prior surgery and is often followed by radiation to the axilla, thus creating a ‘hostile axilla’. An essential step of approaching the axilla as a recipient bed is a full release of the scar that might envelope the recipient vessels, nerves and even tether the muscle. This ensures a healthy bed for lymphangiogenesis and bridging of lymphatics in the recipient bed (Figure 5).
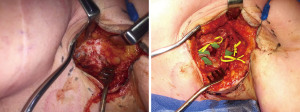
VLNT is reserved for patients with stage 1 and 2 lymphedema, regardless the presence of healthy superficial lymphatic vessels. It can both treat patients with damaged lymphatics or decreased lymph node function and can be combined with LVA, liposuction or a simultaneous breast reconstruction. Alternative donor sites include the lateral thoracic lymph nodes, submental lymph nodes, supraclavicular lymph nodes, and omentum lymph node flap (25,26).
Vascularized groin lymph node flap (VGLNF)
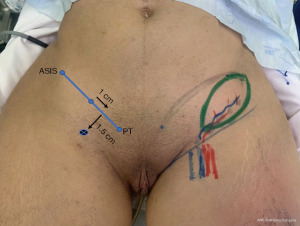
The first and most commonly used option for a lymph node flap is the groin. Becker et al. reported treating upper extremity lymphedema by transferring inguinal lymph nodes to the axillary region (27). The flap is designed in accordance with essential anatomic landmarks and bony prominences described as the golden triangle (28) and it is an ellipse with its central axis parallel to the vascular pedicle [superficial circumflex iliac vein/artery (SCIV/A)]. Increased number and density of the harvested nodes may improve the efficiency of the flap (29). The main concern is that secondary lymphedema may arise in the lower limb after groin VLN harvest. This complication has been reported to be as high as 13.5%, thus extreme caution and profound anatomical knowledge is mandatory for a safer flap design (30). To reduce this hideous complication, it is cardinal to preserve the deeper lymphatics and nodes that are responsible for draining the lower extremity. Harvesting of groin lymph nodes should therefore be superficial to the deep fascia, staying cephalad to the groin crease and lateral to the femoral artery (28). Classically, the reversed lymphatic mapping can be used (31). We currently use a modified reverse lymphatic mapping, using patent blue to map the lower limb draining nodes and distinguish between nodes that drain the limbs and the ones suitable for harvest (32). This gives us a visual advantage during surgery. The golden triangle provides guidelines to optimize the safety (in preventing donor-site lymphedema) and efficacy (enough nodes in the flap) for groin lymph node harvesting (Figure 6). It can also be used for lymph node reconstruction other than breast-cancer related (33).
Lateral thoracic lymph node flap
The second choice for harvesting lymph nodes is the lateral thorax. With anatomical variations, it can be raised based on different pedicles: the lateral thoracic vessels, an accessory lateral thoracic vessel, or a branch of the thoracodorsal artery. Knowing that the number of transplanted lymph nodes correlates positively with an improved lymphatic drainage function, the lateral thoracic donor site, with an average of 13 lymph nodes is considered a good alternative (34). The lateral thoracic artery, however, can be hypoplastic or even absent and then it needs to be harvested on the thoracodorsal artery (35). These high anatomical variations seem to hinder its widespread use. Reverse lymphatic mapping is used to visualize lymphatic drainage of the breast and upper extremity, and aids to ensure a safe flap dissection. This flap possesses favorable features, such as fair pedicle length, abundance of lymph nodes and a cosmetically acceptable donor site scar.
The DIEP flap with lymph nodes
Nowadays, the gold standard for abdominally based autologous reconstruction is a DIEP free flap. After breast cancer treatment, if the patient is not interested in breast reconstruction, a solitary lymph node transfer should suffice. However, some patients require both breast reconstruction and VLNT for treating lymphedema. These patients often have irradiated and scarred axillary region accompanied with lymphedema of the upper limb. This can be addressed with a chimeric flap composed of an abdominal flap for breast reconstruction (DIEP) and a lymph node flap from the groin (VGLNF), targeting both problems in one operation. In this setting, the abdominal flap is based on the deep inferior epigastric vessels and the lymph node flap is based on the superficial circumflex iliac or the superficial inferior epigastric vessels. Using CT scan mapping with measuring distances to anatomic landmarks, the flap is designed in accordance with the safe “golden triangle” zone. The groin lymph nodes are harvested en-bloc with the abdominal flap. When designing the flap, it is recommended to lower the scar down go get better access to the groin and better aesthetic result, much like an aesthetic abdominoplasty. Choosing the perforator for DIEP must consider the breast size. For a small breast, ipsilateral perforator and lymph nodes are harvested and for a bigger breast, a contralateral perforator to the lymph nodes is harvested. In flap setting, if the lymph node harvest in the groin is contralateral to the breast, flipping the flap upside down and setting it in a horizontal fashion is best. When the lymph node harvest is ipsilateral to the breast, setting of the flap is vertical. Placement of the breast reconstruction flap medial on the chest wall and the lymph node flap lateral in the axilla allows using a separate set of anastomoses for each one. It is important to perform an extra anastomosis for the lymph nodes, on top the one to the DIEP and put the nodes in contact with the axillary vein (36). It is crucial to perform extensive scar removal in the axilla releasing vessels and nerves in order to create an open system the flap could integrate in. For the donor-site, seroma formation is a major complication, with higher rates for the combined flap than when harvesting lymph node flap alone. This is due to the big dead space left after a VGLNF and DIEP harvest. Minimizing or avoiding this requires meticulous planning with a prehending reversed lymphatic mapping and use of patent blue. More techniques for reducing seroma include leaving a superior deepithelialized flap for a good closure, using quilting sutures in the groin to minimize dead space, clipping lymphatic vessels, using separate drains for the DIEP and for the groin, donor site compression and the use of fibrin glue (37).
Reductive techniques
Direct excision of the diseased interstitial tissues or skin is an aggressive measure that in western population, is rarely used, if ever, for the upper limb due to its mutilating and disfiguring effect.
Liposuction is a method that permits effective volume reduction in therapy-resistant lymphedema of the limbs. It is performed using power-assisted liposuction aimed at removing the pathological hypertrophied fat from the interstitium. A tourniquet and tumescent can be used to minimize blood loss. Described by Brorson (38), full liposuction is performed circumferentially from wrist to shoulder and was demonstrated to be an effective method for the treatment of chronic, nonpitting, arm lymphedema resistant to conservative treatment (39). It is reserved for patients with an important circumferential fat hypertrophy of the limb. When residual pitting edema is seen in limb, selective liposuction can be performed in specific resistant regions to reduce adipose tissue deposits. It is essential to catch the disease on time, before it progresses irreversibly and liposuction is the only possibility. Those who present with advanced stage or fail conservative therapy and are not candidates for microsurgical reconstructive surgery may benefit from liposuction. It will provide volume reduction and diminished circumference of the limb, but once performed, liposuction mandates the patient to wear lifelong compression garments. In selected cases, 6–12 months after liposuction was done, an additional lymph node transfer can be performed in order to stabilize the result.
Treatment algorithm
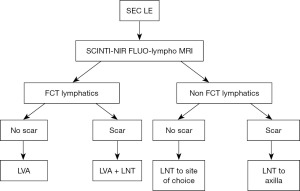
The approach to lymphatic limb surgery should focus on tailoring the best matched procedure to each patient. Lymphedema patients with pitting lymphedema are worked up by preoperative imaging with lymphoscintigraphy and near infra-red fluorescence, and in recent years lympho-MRI has been incorporation for selected cases. When lymphatics are functional, the patient is a candidate to perform lympho-venous anastomosis (LVA). When there is no scar in the root of the limb, the axilla, LVA alone is performed. In the presence of a scar, lymph nodes transplantation to the debrided scar is combined with LVA distally. In the event there are no functional lymphatics in a patient with pitting lymphedema and there is no scar, lymph node transplantation in performed to the site of choice, either anatomic or non-anatomic. When the axilla is scarred however, the root of the limb must be addressed and then the lymph node transplantation goes to the axilla. This protocol is described in Figure 7.
Post-surgery care
Post-op treatment is again tailored specifically to each patient and her needs. Remembering that lymphedema is a progressive chronic disease with its ups and downs might require adjustments, even after a corrective surgery. Compression garments are advised and CTD and manual lymphatic drainage are started 10 days after surgery. We continue routine follow-ups, making and recording measurements using the Perometer. The same preventive measures as for each lymphedema patient are suggested (skin care, infection prevention and hygiene, no direct shocks to the affected limb, etc.).
A possible adjunct in the follow up of patients is the low energy extracorporeal shockwave therapy (ESWT). These are acoustic, electromagnetic pulses transmitted into the human tissue inducing an intracellular biological reaction (40). It was demonstrated to stimulate angiogenesis and lymphangiogenesis, reducing inflammatory response and upregulating cell proliferation (41). This method is as an alternative noninvasive treatment for residual, end-stage, secondary upper limb lymphedema. It has been demonstrated that upper limb circumference measurements were significantly reduced after 4 weeks of treatment (42). This is also in concordance with studies that reported of 30% and even higher mean total circumference reduction (43). Given this data, ESWT can be considered an additional treatment option to improve the clinical outcome of refractory, long-standing secondary lymphedema.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard and Jørn Bo Thomsen) for the series “Breast Reconstruction—The True Multidisciplinary Approach” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-154/coif). The series “Breast Reconstruction-The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cheville AL, Beck LA, Petersen TL, et al. The detection and treatment of cancer-related functional problems in an outpatient setting. Support Care Cancer 2009;17:61-7. [Crossref] [PubMed]
- McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Patient perceptions and precautionary behaviors. J Clin Oncol 2008;26:5220-6. [Crossref] [PubMed]
- Salinas-Huertas S, Luzardo-González A, Vázquez-Gallego S, et al. Risk factors for lymphedema after breast surgery: A prospective cohort study in the era of sentinel lymph node biopsy. Breast Dis 2022;41:97-108. [Crossref] [PubMed]
- Boneti C, Korourian S, Bland K, et al. Axillary reverse mapping: mapping and preserving arm lymphatics may be important in preventing lymphedema during sentinel lymph node biopsy. J Am Coll Surg 2008;206:1038-42; discussion 1042-4. [Crossref] [PubMed]
- Hoe AL, Iven D, Royle GT, et al. Incidence of arm swelling following axillary clearance for breast cancer. Br J Surg 1992;79:261-2. [Crossref] [PubMed]
- Duff M, Hill AD, McGreal G, et al. Prospective evaluation of the morbidity of axillary clearance for breast cancer. Br J Surg 2001;88:114-7. [Crossref] [PubMed]
- International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology 2013;46:1-11. [PubMed]
- Mihara M, Hara H, Hayashi Y, et al. Pathological steps of cancer-related lymphedema: histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One 2012;7:e41126. [Crossref] [PubMed]
- Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 2013;132:1305-14. [Crossref] [PubMed]
- Greene AK. Diagnosis and Management of Obesity-Induced Lymphedema. Plast Reconstr Surg 2016;138:111e-8e. [Crossref] [PubMed]
- Javid SH, Anderson BO. Mounting evidence against complex decongestive therapy as a first-line treatment for early lymphedema. J Clin Oncol 2013;31:3737-8. [Crossref] [PubMed]
- Bourgeois P, Leduc O, Leduc A. Imaging techniques in the management and prevention of posttherapeutic upper limb edemas. Cancer 1998;83:2805-13. [Crossref] [PubMed]
- Yamamoto T, Yamamoto N, Doi K, et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: a novel severity staging system using dermal backflow patterns. Plast Reconstr Surg 2011;128:941-7. [Crossref] [PubMed]
- Narushima M, Yamamoto T, Ogata F, et al. Indocyanine Green Lymphography Findings in Limb Lymphedema. J Reconstr Microsurg 2016;32:72-9. [PubMed]
- Notohamiprodjo M, Weiss M, Baumeister RG, et al. MR lymphangiography at 3.0 T: correlation with lymphoscintigraphy. Radiology 2012;264:78-87. [Crossref] [PubMed]
- Liu NF, Lu Q, Jiang ZH, et al. Anatomic and functional evaluation of the lymphatics and lymph nodes in diagnosis of lymphatic circulation disorders with contrast magnetic resonance lymphangiography. J Vasc Surg 2009;49:980-7. [Crossref] [PubMed]
- Zeltzer AA, Brussaard C, Koning M, et al. MR lymphography in patients with upper limb lymphedema: The GPS for feasibility and surgical planning for lympho-venous bypass. J Surg Oncol 2018;118:407-15. [Crossref] [PubMed]
- Masia J, Kosutic D, Clavero JA, et al. Preoperative computed tomographic angiogram for deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg 2010;26:21-8. [Crossref] [PubMed]
- Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg 2000;16:437-42. [Crossref] [PubMed]
- Campisi C, Bellini C, Campisi C, et al. Microsurgery for lymphedema: clinical research and long-term results. Microsurgery 2010;30:256-60. [Crossref] [PubMed]
- Boccardo F, Casabona F, De Cian F, et al. Lymphedema microsurgical preventive healing approach: a new technique for primary prevention of arm lymphedema after mastectomy. Ann Surg Oncol 2009;16:703-8. [Crossref] [PubMed]
- Boccardo F, Casabona F, De Cian F, et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: over 4 years follow-up. Microsurgery 2014;34:421-4. [Crossref] [PubMed]
- Patel KM, Lin CY, Cheng MH. From theory to evidence: long-term evaluation of the mechanism of action and flap integration of distal vascularized lymph node transfers. J Reconstr Microsurg 2015;31:26-30. [Crossref] [PubMed]
- Cheng MH, Chen SC, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg 2013;131:1286-98. [Crossref] [PubMed]
- Nguyen AT, Suami H, Hanasono MM, et al. Long-term outcomes of the minimally invasive free vascularized omental lymphatic flap for the treatment of lymphedema. J Surg Oncol 2017;115:84-9. [Crossref] [PubMed]
- Maldonado AA, Chen R, Chang DW. The use of supraclavicular free flap with vascularized lymph node transfer for treatment of lymphedema: A prospective study of 100 consecutive cases. J Surg Oncol 2017;115:68-71. [Crossref] [PubMed]
- Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg 2006;243:313-5. [Crossref] [PubMed]
- Zeltzer AA, Anzarut A, Braeckmans D, et al. The vascularized groin lymph node flap (VGLN): Anatomical study and flap planning using multi-detector CT scanner. The golden triangle for flap harvesting. J Surg Oncol 2017;116:378-83. [Crossref] [PubMed]
- Patel KM, Chu SY, Huang JJ, et al. Preplanning vascularized lymph node transfer with duplex ultrasonography: an evaluation of 3 donor sites. Plast Reconstr Surg Glob Open 2014;2:e193. [Crossref] [PubMed]
- Vignes S, Blanchard M, Yannoutsos A, et al. Complications of autologous lymph-node transplantation for limb lymphoedema. Eur J Vasc Endovasc Surg 2013;45:516-20. [Crossref] [PubMed]
- Yue T, Zhuang D, Zhou P, et al. A Prospective Study to Assess the Feasibility of Axillary Reverse Mapping and Evaluate Its Effect on Preventing Lymphedema in Breast Cancer Patients. Clin Breast Cancer 2015;15:301-6. [Crossref] [PubMed]
- Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015;135:277-85. [Crossref] [PubMed]
- Zeltzer AA, Anzarut A, Hamdi M. A Review of Lymphedema for the Hand and Upper-Extremity Surgeon. J Hand Surg Am 2018;43:1016-25. [Crossref] [PubMed]
- Nguyen DH, Chou PY, Hsieh YH, et al. Quantity of lymph nodes correlates with improvement in lymphatic drainage in treatment of hind limb lymphedema with lymph node flap transfer in rats. Microsurgery 2016;36:239-45. [Crossref] [PubMed]
- Gerety PA, Pannucci CJ, Basta MN, et al. Lymph node content of supraclavicular and thoracodorsal-based axillary flaps for vascularized lymph node transfer. J Vasc Surg Venous Lymphat Disord 2016;4:80-7. [Crossref] [PubMed]
- Shesol BF, Nakashima R, Alavi A, et al. Successful lymph node transplantation in rats, with restoration of lymphatic function. Plast Reconstr Surg 1979;63:817-23. [Crossref] [PubMed]
- Hamdi M, Ramaut L, De Baerdemaeker R, et al. Decreasing donor site morbidity after groin vascularized lymph node transfer with lessons learned from a 12-year experience and review of the literature. J Plast Reconstr Aesthet Surg 2021;74:540-8. [Crossref] [PubMed]
- Brorson H. Liposuction in Lymphedema Treatment. J Reconstr Microsurg 2016;32:56-65. [PubMed]
- Hoffner M, Ohlin K, Svensson B, et al. Liposuction Gives Complete Reduction of Arm Lymphedema following Breast Cancer Treatment-A 5-year Prospective Study in 105 Patients without Recurrence. Plast Reconstr Surg Glob Open 2018;6:e1912. [Crossref] [PubMed]
- Wang CJ, Wang FS, Yang KD, et al. Shock wave therapy induces neovascularization at the tendon-bone junction. A study in rabbits. J Orthop Res 2003;21:984-9. [Crossref] [PubMed]
- Yan X, Zeng B, Chai Y, et al. Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low-energy shockwave therapy in random-pattern skin flap model. Ann Plast Surg 2008;61:646-53. [Crossref] [PubMed]
- Joos E, Vultureanu I, Nonneman T, et al. Low-Energy Extracorporeal Shockwave Therapy as a Therapeutic Option for Patients with a Secondary Late-Stage Fibro-Lymphedema After Breast Cancer Therapy: A Pilot Study. Lymphat Res Biol 2021;19:175-80. [Crossref] [PubMed]
- Cebicci MA, Sutbeyaz ST, Goksu SS, et al. Extracorporeal Shock Wave Therapy for Breast Cancer-Related Lymphedema: A Pilot Study. Arch Phys Med Rehabil 2016;97:1520-5. [Crossref] [PubMed]
Cite this article as: Tobias T, Zeltzer AA. Current concepts of lymphedema treatment for the breast cancer patient: a clinical practice review. Ann Breast Surg 2023;7:8.