
The neoadjuvant systemic treatment of early breast cancer: a narrative review
Introduction
Over the last decade, the use of neoadjuvant chemotherapy (NACT) for the treatment of early breast cancer, particularly locally advanced breast cancers, has significantly increased (1). There is a growing need for all disciplines involved in the treatment of early breast cancer to understand and discuss with patients the potential role of neoadjuvant treatment for their tumor subtype. Results of the NSABP-B18 trial demonstrated that NACT provides similar disease-free survival (DFS) and overall survival (OS) as adjuvant chemotherapy. Patients receiving NACT experienced an increased likelihood of breast conserving surgery (BCS) and of pathologically negative nodes (2). A 2018 meta-analysis by the early breast cancer trialists collaborative group (EBCTCG) showed that NACT increases BCS rates compared to adjuvant chemotherapy (3). NACT may also spare clinically node positive patients the long-term morbidity associated with an axillary lymph node dissection (ALND). The SENTINA (4) and ACOSOG Z1071 trial (5) showed that clinically node positive patients who respond to NACT can be accurately staged by sentinel lymph node biopsy (SLNB) alone and the use of dual tracers and removal of at least three sentinel nodes provides a clinically acceptable false negative rate (FNR) of <10%, while placing a clip in the biopsy proven node and removing it at surgery further reduces the FNR (6). Beyond surgical advantages, neoadjuvant systemic therapy provides important prognostic information. A pooled analysis of 12 neoadjuvant trials involving almost 12,000 patients showed that on an individual patient level a pathological complete response (pCR) defined as no residual invasive tumor in the breast or lymph nodes was significantly associated with event free survival (EFS) and OS (7). This association was strongest in patients with human epidermal growth factor receptor-2 (HER-2) positive and triple negative (TN) tumors compared to hormone receptor (HR) positive tumors. While pCR is dichotomous, a graded index known as the residual cancer burden (RCB) has also been shown to be prognostic of long term survival (8) with pCR classified as RCB-0, minimal residual disease as RCB-1, moderate residual disease as RCB-2 and extensive residual disease as RCB-3 (9). This index has also been shown to be continuously prognostic independent of other clinicopathological variables for 10-year relapse free survival in all 3 breast cancer subtypes, with a greater prognostic impact in the TN and HER-2 positive subtypes (8). The prognostic insight provided by pCR has been translated into positive postoperative treatment escalation studies using residual disease to predict which patients may benefit from additional postoperative systemic therapy (10,11). The primary surgical and oncological advantages of neoadjuvant systemic treatment are shown in Table 1. Ancillary advantages of neoadjuvant treatment include increased time for genetic testing and consideration of reconstructive or prophylactic surgical options prior to breast surgery. Despite the adoption of a multidisciplinary approach to the treatment of early breast cancer, for many patients it is still unclear who stands to benefit most from a neoadjuvant approach, limiting its clinical implementation. In this review we aim to provide all clinicians involved in the treatment of early breast cancer with a comprehensive assessment of the role of neoadjuvant systemic therapy in HR positive, HER-2 positive and TN breast cancer, focusing on patient selection, surgical and oncological benefits, and future directions. We present the following article in accordance with the Narrative Review reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-109/rc).
Table 1
| 1. Improve patient DFS and OS similarly to adjuvant therapy |
| 2. Improve surgical outcomes (breast conservation rates, spare axillary dissection) |
| 3. Provide prognostic information |
| 4. Enable escalation or de-escalation of postoperative systemic treatment |
DFS, disease-free survival; OS, overall survival.
Methods
We performed a search of the PubMed, Cochrane Review, and Clinical Trials.gov databases. Only English language publications were included. The search terms were as follows: “neoadjuvant chemotherapy” AND “breast cancer”. We conducted a thorough manual review of all bibliographies and relevant studies to identify additional potentially eligible studies (Table 2).
Table 2
| Items | Specification |
|---|---|
| Date of search | July 2021 repeated March 2022 for updated data |
| Databases and other sources searched | PubMed, Cochrane Review, Clinical Trials.gov |
| Search terms used | Neoadjuvant chemotherapy and breast cancer |
| Timeframe | From July 1997 to February 2022 |
| Inclusion and exclusion criteria | English only |
| Selection process | Selection conducted by all authors together |
| Additional considerations | A manual review of bibliographies identified additional relevant studies; newly published data was updated during the manuscript writing process |
HER-2 positive breast cancer
Anti-HER-2 therapy administered with chemotherapy in patients with HER-2 positive breast cancer has led to a significant reduction in tumor recurrence and death, and when given preoperatively, is associated with high rates of pCR (12,13). Nevertheless, not all patients require such intensive treatment and de-escalation of anti-HER-2 targeted therapies and chemotherapy in appropriately selected populations has been an area of increased research. Currently, patients with stage 1 HER-2 positive breast cancer have excellent outcomes with adjuvant single agent paclitaxel and trastuzumab (14) and unless breast tumor downstaging is required to optimize surgery these patients do not require neoadjuvant treatment. Neoadjuvant treatment can be considered in all medically fit patients with stage 2 or 3 HER-2 positive breast cancer regardless of their pretreatment eligibility for BCS as their response to neoadjuvant treatment may affect postoperative treatment decisions (15).
Pivotal trials
The adjuvant NSABP B-31/NCCTG-N9831 trials demonstrated that the addition of one year of trastuzumab to anthracycline/taxane based chemotherapy resulted in a 40% reduction in breast cancer recurrences and a 37% reduction in mortality (12). Following the success of trastuzumab in the adjuvant setting, phase 2 trials showed impressive pCR rates in the neoadjuvant setting in patients with stage 2 and 3 HER-2 positive breast cancer treated with trastuzumab and chemotherapy (15-17). In the phase 3 NOAH (Neoadjuvant Herceptin) trial the addition of trastuzumab to chemotherapy yielded a response rate of 81% and a significantly superior pCR rate compared to chemotherapy alone (13) translating into a 36% relative improvement in 5-year EFS (18). The HER-2 dimerization inhibitor pertuzumab further improved outcomes when incorporated into both neoadjuvant and adjuvant trastuzumab/chemotherapy regimens and received accelerated approval in the neoadjuvant setting based largely on data from the phase 2 NeoSphere trial (19,20). This trial compared pCR rates between docetaxel/trastuzumab/pertuzumab (THP), docetaxel/trastuzumab (TH), docetaxel/pertuzumab (TP) and trastuzumab/pertuzumab (HP) in patients with stage 2 or 3 HER-2 positive breast cancer. All patients received additional anthracycline-based chemotherapy after surgery, regardless of response. Among arms, the THP combination was superior and demonstrated a pCR rate of 46%. Notably, even with the combination of trastuzumab and pertuzumab, without chemotherapy, 17% of patients experienced pCR, suggesting that for selected patients, treatment may potentially be de-escalated to exclude chemotherapy (20). To spare patients the potential long-term cardiac and myelotoxicity of anthracycline based regimens, the phase 2 TRYPHAENA trial examined the safety and efficacy of the anthracycline-free regimen TCHP (docetaxel, carboplatin, trastuzumab, pertuzumab) (21). This combination yielded a pCR rate of 66% with fewer declines in left ventricular ejection fraction compared to the anthracycline-based regimens. Further evidence supporting the use of an anthracycline-free regimen comes from the phase 3 TRAIN-2 trial demonstrating that a neoadjuvant platinum/taxane based regimen in combination with trastuzumab and pertuzumab provides equivalent 3-year EFS rates compared to a traditional anthracycline containing regimen (22). Overall, these pivotal neoadjuvant trials in HER-2 positive breast cancer show that between 50–80% of patients with HER-2 positive tumors will experience pCR following NACT with dual anti-HER-2 blockade and approximately 90% of patients who experience pCR will remain disease free 4 years after surgery (23).
Postoperative/adjuvant treatment escalation
Despite the significant improvements described above, between 20–50% of patients do not experience pCR. This patient population is at a higher risk for disease recurrence and death (HR with pCR, EFS: 0.39, 95% CI: 0.31–0.5; OS: 0.34, 95% CI: 0.24–0.47) (7) and thus warrants modification of the postoperative adjuvant therapy. The phase 3 KATHERINE trial randomized 1,486 HER-2 positive patients with residual disease following NACT and trastuzumab (approximately 18% in each arm received pertuzumab as well) to either standard adjuvant trastuzumab or T-DM1 [an antibody drug conjugate of trastuzumab (T) and the cytotoxic agent emtansine (DM1)] to complete 1 year of treatment. Patients receiving T-DM1 experienced a significant reduction in 3-year invasive disease-free survival (iDFS) (88.3% vs. 77%, P<0.001) (11). Given the results of this trial, neoadjuvant treatment in HER-2 positive breast cancer is now indicated not only to improve surgical outcomes and provide prognostic information, but also to predict a benefit for switching treatment from trastuzumab to T-DM1 in the postoperative setting. While the seminal neoadjuvant trials included only patients with stage 2 or 3 breast cancer the KATHERINE trial also included a small number of patients with stage 1 disease, suggesting a potential benefit of neoadjuvant treatment in this population as well. In the final efficacy results of the ExteNET trial which examined the role of 1 year of adjuvant treatment with the pan-HER tyrosine kinase inhibitor neratinib after one year of trastuzumab; among 295 HR positive patients with residual disease post-NACT, one year of neratinib resulted in a 9.1% improvement in 8-year OS (91.3% vs. 82.2%, P=0.031) (24). These results are yet another example of how a HER-2 targeted agent can be personally tailored to improve patient outcomes based on their response to neoadjuvant treatment.
De-escalating treatment
As described, some patients have excellent responses to anti-HER-2 antibodies with single agent chemotherapy or without chemotherapy altogether, setting the stage for potential strategies for de-escalation of toxic chemotherapy in the neoadjuvant setting. The patient sub-groups that benefit from de-escalation still need to be defined (see biomarker discussion below). The ongoing Compass and Decrescendo trials are examining whether single agent taxane plus dual HER-2 inhibition with THP given for 4 cycles will be sufficient in patients who experience pCR (25,26). Patients with residual disease at surgery will receive adjuvant T-DM1 ± additional chemotherapy per investigator’s choice. The KRISTINE trial which randomized 444 patients with stage 2–3 HER-2 positive breast cancer to 6 cycles of neoadjuvant T-DM1 with pertuzumab or TCHP showed inferior pCR rates and increased rates of locoregional progression before surgery with T-DM1 (27). With the results of this trial, the use of T-DM1 in the neoadjuvant setting has not been incorporated into standard clinical practice.
Biomarkers for response
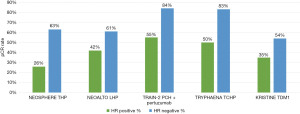
De-escalation strategies should optimally rely on biomarkers for response to the targeted treatment. One possible biomarker is HR negativity as it is consistently correlated with superior pCR rates in HER-2 positive breast cancer (Figure 1). For example, in NeoSphere patients with HR negative disease treated with THP or the chemotherapy free HP combination had pCR rates of 63% and 27% respectively (20). In TRYPHAENA, HR negative patients receiving TCHP had a pCR rate of 83% compared to 50% among those with HR positive tumors (12). Lastly, in the phase 2 West German Study Group (WSG) ADAPT trial HER-2 positive HR negative patients were randomized to trastuzumab with pertuzumab ± paclitaxel. For the 42 patients receiving paclitaxel with trastuzumab and pertuzumab the pCR rate was 90.5% (28). Another potential predictor of response to anti-HER-2 treatment is intratumoral HER-2 heterogeneity. Filho et al. explored the role of intratumoral HER-2 heterogeneity in a single arm phase 2 trial of neoadjuvant T-DM1 with pertuzumab (29). Patients were biopsied in 2 different areas of the tumor with 3 cores taken from each area. Intratumoral HER-2 heterogeneity was defined as at least one of the six cores demonstrating either HER-2 positivity by flourescence in situ hybridization (FISH) in >5% and <50% of tumor cells or an area of tumor that tested HER-2 negative. Among 164 patients enrolled, none of the patients with HER-2 heterogenous tumors experienced pCR, suggesting that these patients may not be appropriate candidates for omission of chemotherapy. Molecular subtyping may also provide additional predictive information; in the phase 2 PAMELA trial 151 patients with HER-2 positive stage 1–3 breast cancer were treated with dual anti HER-2 blockade using lapatinib and trastuzumab for 18 weeks and the association between molecular subtype as defined by the PAM50 assay and pCR was evaluated (30). In this study 101/151 (67%) of the HER-2 positive patients were of the HER-2 enriched subtype. Notably 41% of these had pCR at surgery while 10% of the non-HER-2 enriched tumors showed a pCR.
While these biomarkers are promising, until large randomized trials provide definitive evidence that certain populations can be spared multiagent chemotherapy without compromising long term outcomes, patients with HER-2 positive breast cancer undergoing neoadjuvant treatment should receive standard anthracycline based or platinum/taxane based chemotherapy combined with dual anti HER-2 inhibition with trastuzumab and pertuzumab (15).
Triple negative breast cancer (TNBC)
HR negative and HER-2 negative breast cancer known as TNBC composes approximately 15% of all cases of breast cancer, is more commonly diagnosed in women younger than 40 years and is considered to be more aggressive with worse prognosis (31,32). NACT may be offered to all chemotherapy eligible TNBC patients with tumors above 2 cm or positive lymph nodes, regardless of BCS eligibility (15). A pCR following NACT is of particular significance in TNBC as the association between long-term outcomes is strongest in this patient population (HR for EFS with pCR 0.24, 95% CI: 0.18–0.33) (7).
Historically anthracycline/taxane based regimens have been preferred in the treatment of TNBC (32). In the adjuvant setting in the combined analysis of the Anthracycline in Breast Cancer (ABC) trials the anthracycline-free regimen of docetaxel and cyclophosphamide (TC) was found to be inferior to standard anthracycline/taxane based chemotherapy, particularly for patients with TNBC or positive lymph nodes, reinforcing the continued role of anthracyclines in TNBC (33). In contrast in the WSG Plan B study adjuvant TC was found to be noninferior to a standard anthracycline/taxane regimen regardless of HR expression or lymph node status (34). In the neoadjuvant setting, there are some data suggesting that a taxane/platinum combination may provide similar pCR rates to the anthracycline/taxane based regimens. Sharma et al. (35) reported that pCR rate was 55% following NACT with docetaxel and carboplatin concluding that this regimen yields promising efficacy. Further support is seen in the phase 2 NeoSTOP trial where patients randomized to receive 6 cycles of docetaxel and carboplatin demonstrated an identical pCR rate as those that received 4 cycles of paclitaxel and carboplatin followed by 4 cycles of doxorubicin and cyclophosphamide (36). However, large neo-adjuvant trials comparing these regimens with EFS as an endpoint are lacking. Thus, anthracycline containing NACT regimens remain the standard in TNBC. For patients who are not eligible for anthracyclines due to a history of cardiac disease or major risk factors for cardiac toxicity the use of an anthracycline free regimen may be warranted.
The order and nature of the taxane
It appears that the sequence of treatment does not matter and the anthracyclines can either be followed or preceded by a taxane (37). In addition, there is no overwhelming evidence that the nature of the taxane influences outcomes (38). In the adjuvant setting weekly or every 2 weeks solvent based paclitaxel appears to have the most efficacy (38,39) While nab-paclitaxel (nanoparticle albumin bound paclitaxel) has shown superiority to solvent-based paclitaxel in some studies, others have failed to show a significant difference. GeparSepto demonstrated improved pCR with nab-paclitaxel compared to paclitaxel in all breast cancer subtypes including TNBC (pCR entire cohort 38.4% vs. 29%, P=0.00065, TNBC 48% vs. 26%, P=0.00027) (40). This improvement in pCR translated to a significantly improved 4-year iDFS (41). In contrast, the ETNA trial which also compared these 2 taxanes in the neoadjuvant setting failed to show a significant difference in pCR rates (42).
Addition of carboplatin
The addition of carboplatin to standard anthracycline/taxane based NACT in TNBC is controversial. A meta-analysis of 9 randomized clinical trials including 2,109 patients found that the addition of platinum increased pCR rates significantly from 37% to 52.1% (P<0.001) with an increase in hematological toxicity (43). While effectively increasing pCR, its effect on long-term outcomes is uncertain. In GeparSixto the addition of carboplatin to the anthracycline/taxane backbone significantly improved pCR rates (53.2% vs. 36.9%, P=0.005) translating into an improvement in 3-year DFS (86% vs. 76%, P=0.022) (44,45). In contrast the addition of carboplatin to doxorubicin and paclitaxel in CALGB 40603 provided similar improvements in pCR yet failed to demonstrate an improvement in DFS (46,47). Notably, in GeparSixto patients with germline BRCA1/2 mutations did not experience improvements in pCR from the addition of carboplatin with exceptional pCR rates irrespective of carboplatin treatment (48). While current guidelines allow for the consideration of carboplatin as part of the neoadjuvant treatment of TNBC (15), the lack of definitive data demonstrating its effect on long term outcomes has prevented it from becoming a standard of care worldwide.
Addition of immune checkpoint inhibitors
Programmed cell death 1 (PD-1) is a transmembrane protein expressed on T cells, B cells, and NK cells. This protein binds to PD-1 ligand (PD-L1) and has an inhibitory effect, particularly on cytotoxic T cells (49). PD-L1 is expressed on the surface of multiple tissue types, including tumor cells and tumor infiltrating immune cells (50). Inhibition of the interaction between PD-1 to PD-L1 may restore the ability of T cells to identify and attack cancer cells (49). Various immune check point inhibitors (CPI) inhibiting PD-1 (pembrolizumab, nivolumab) or PD-L1 (atezolizumab, avelumab, durvalumab) have been approved for use in various tumor types. TNBC is considered the most immunogenic of all the breast cancer subtypes (51) and in the metastatic setting the combination of a CPI with chemotherapy has been shown to improve progression free survival (PFS) and OS in patients expressing PD-L1 on tumor cells or tumor infiltrating immune cells (52,53).
The beneficial role of the addition of CPI to NACT in TNBC is currently unfolding. The phase 3 KEYNOTE-522 trial examined the effect of adding pembrolizumab to an anthracycline/taxane based regimen including carboplatin in the neoadjuvant setting. At the first interim analysis the addition of pembrolizumab showed a 13.6% improvement in pCR (64.8% vs. 51.5%, P=0.00055) (54). A recently reported analysis of 3-year EFS demonstrated a significant improvement in favor of patients who received pembrolizumab (84.5% vs. 76.8%, P=0.00031) (55). In an exploratory subgroup analysis based on response to neoadjuvant treatment, patients who experienced pCR in both groups had excellent 3-year EFS outcomes [94.4% vs. 92.5%, P value not reported (NR)] while the patients who did not experience pCR appeared to derive a clinically significant benefit from the addition of pembrolizumab to the NACT regimen (3-year EFS: 67.4% vs. 56.8%, P value NR) (55). Based on these latest results the FDA has recently approved the use of pembrolizumab combined with NACT for neoadjuvant treatment of high risk TNBC. The phase 3 IMpassion031 trial examined the effect of adding atezolizumab to anthracycline/taxane based NACT without carboplatin. The addition of atezolizumab significantly increased pCR by 17% (58% vs. 41%, P=0.0044). EFS and OS results are immature (56). Smaller phase 2 trials have shown mixed results with CPI in the neoadjuvant setting. Both NeoTRIPaPDL1 which examined the addition atezolizumab to NACT and GeparNuevo which examined the addition of durvalumab to NACT did not demonstrate a statistically significant improvement in pCR (57,58), however the long term results of the GeparNuevo trial demonstrated a significant improvement in both DFS and OS despite the modest improvement in pCR (59). Thus, while pCR rates are highly correlated to prognosis after NACT treatment the correlation of pCR with neoadjuvant CPI treatment is not as clear. Importantly, CPI treatment may be associated with potentially severe and sometimes long-term toxicity, particularly endocrinopathies requiring lifelong medication (60). As more long-term results become available in the next year, we expect that CPIs will be regularly incorporated into the neoadjuvant treatment regimens of TNBC.
Addition of poly-ADP-ribose polymerase (PARP) inhibitors
Up to 20% of patients with TNBC harbor a germline BRCA1/2 mutation (61). Carriers of deleterious BRCA1/2 mutations lose expression or function of BRCA1/2 proteins in cancer cells resulting in damage to the homologous DNA repair mechanism responsible for repairing double strand DNA breaks (62). The PARP are key players in repair of DNA single strand breaks (63). PARP inhibitors (PARPi) promote death of BRCA deficient cells by a “synthetic lethality” mechanism. These drugs prevent repair of single DNA strand breaks eventually causing accumulation of double strand breaks. In tumors without proper function of BRCA proteins these double strand breaks cannot be repaired causing death of the cancer cells (64).
PARPi are used in the treatment of metastatic breast cancer patients who carry a germline BRCA 1/2 mutation where they improved PFS (65,66) and possibly OS when used in first line (67). Recently, the phase 3 Olympia trial demonstrated that 1 year of adjuvant Olaparib significantly improves 3-year DFS (85.9% vs. 77.1%, P<0.001) in germline BRCA1/2 mutant breast cancer patients with at least stage 2 tumors that did not receive NACT or did not experience pCR following NACT (68).
The role of PARPi in the neoadjuvant setting is currently being explored. The adaptive phase 2 ISPY2 trial demonstrated that adding carboplatin and the PARPi veliparib to standard anthracycline/taxane NACT improved pCR compared to the standard anthracycline/taxane alone in patients with TNBC (51% vs. 26%, P value NR) (69). These results led to the phase 3 BrighTNEss trial which randomized 634 patients (15% germline BRCA 1/2 mutation) to either neoadjuvant paclitaxel plus carboplatin plus veliparib, paclitaxel plus carboplatin or paclitaxel alone. After receiving one of these three regimens all patients received 4 cycles of anthracycline based treatment. While both the carboplatin-veliparib combination and carboplatin monotherapy arms achieved increased pCR rates compared to paclitaxel alone, the addition of veliparib failed to improve pCR beyond that of carboplatin alone (70); suggesting that PARPi may not have a role in the neoadjuvant setting in patients already receiving a platinum agent. In a small study by Litton et al. (71) 10 out of 19 (53%) patients carrying germline BRCA 1/2 mutations who received single agent talazoparib for 6 months had a pCR. In the phase 2 NEOTALA study of 48 evaluable TNBC patients with germline BRCA1/2 mutations 45.8% demonstrated a pCR after 24 weeks of talazoparib treatment (72). These data are promising and various larger clinical trials using neoadjuvant PARPi as single agents or in combination with CPIs are planned. Use of neoadjuvant PARPi outside of clinical trials is currently not recommended.
The pCR rates for the major TNBC neoadjuvant trials are summarized in Table 3.
Table 3
| Study | Study design | pCR | Treatment arms | Number of TNBC patients | pCR | P value | DFS/EFS | P value |
|---|---|---|---|---|---|---|---|---|
| GeparSixto (44,45) | Phase II | ypT0pN0 | P + Dox + Bev + Cb | 158 | 53.2% | 0.005 | 86.1% | 0.0224 |
| P + Dox + Bev | 157 | 36.9% | 75.8% | |||||
| CALGB 40603 (46,47) | Phase II | ypT0/is | P + Cb → ddAC ± Bev | 221 | 60% | 0.0018 | NR | |
| P → ddAC ± Bev | 212 | 46% | NR | |||||
| Keynote 522 (54,55) | Phase III | ypT0/TisypN0 | Pembrolizumab + P + Cb → AC | 784 | 64.8% | <0.001 | 84.3% | 0.0003 |
| Placebo + P + Cb → AC | 390 | 51.2% | 76.2% | |||||
| IMpassion031 (56) | Phase III | ypT0/is ypN0 | Atezolizumab + NabP → AC | 165 | 58% | 0.0044 | Immature | |
| Placebo + NabP → AC | 168 | 41% | Immature | |||||
| GeparNuevo (58,59) | Phase II | ypT0 ypN0 | Durvalumab + NabP → EC + durvalumab | 88 | 53.4% | 0.224 | 85.6% | 0.0398 |
| Placebo + NabP → EC + placebo | 86 | 44.2% | 77.2% | |||||
| BrighTNess (70) | Phase III | ypT0pN0 | P + Cb + veliparib → AC | 316 | 53% | 0.36* | 78% | |
| P + Cb + placebo → AC | 160 | 58% | <0.001** | 79% | ||||
| P + placebo → AC | 158 | 31% | 69% | 0.02 |
*, P + Cb + veliparib vs. P + Cb; **, P + Cb + veliparib vs. P + placebo. pCR, pathological complete response; TNBC, triple negative breast cancer; DFS, disease-free survival; EFS, event free survival; P, paclitaxel; Dox, doxorubicin; Bev, bevaciumab; Cb, carboplatin; dd, dose dense; AC, adriamycin-cyclophosphamide; NR, not reported; NabP, nabpaclitaxel; EC, epirubicin + cyclophosphamide.
Post-operative treatment for patients not achieving pCR
The CREATE-X trial randomly assigned 910 patients with HER-2-negative residual invasive breast cancer after NACT to postsurgical treatment with capecitabine or placebo. Among patients with TNBC, the addition of capecitabine significantly improved DFS and OS (10). Similar to the KATHERINE trial in HER-2 positive patients (11) and Olympia in germline BRCA1/2 related breast cancer (68), CREATE-X demonstrated how postoperative treatment can be tailored to improve outcomes based on the response to NACT in TNBC.
HR positive breast cancer
NACT
While chemotherapy in HER-2 positive and TNBC is routinely used, the decision to administer NACT in HR positive breast cancer is more complex, as many patients are not expected to derive a significant survival benefit from chemotherapy (73). While it has been reported that following NACT over 70% of HR positive patients have a clinical and pathological response in the breast and up to 21.1% have been shown to have a complete pathological axillary response (74), it is still unclear which patients will be able to avoid mastectomy or the sequelae of an ALND (75) after NACT. The pCR rates are very low, with an expected rate of less than 10% in low grade tumors and less than 20% in high grade tumors (7). Moreover, the prognostic value of pCR in HR positive disease is questionable, especially in low grade luminal A like disease [defined clinically as high estrogen receptor (ER) and progesterone receptor (PR) levels, negative HER-2 and Ki-67 <15%] indicating a need for better pathologic response measures of neoadjuvant treatment in this patient population (76). Efforts have been made to define HR positive subgroups that will derive benefit from NACT. Gene expression profiles such as Oncotype Dx and MammaPrint, commonly used to support adjuvant chemotherapy decision-making in HR positive breast cancer are being explored in the neoadjuvant setting. There is a growing amount of evidence showing the concordance of gene expression profiles derived from preoperative core needle biopsies to surgical specimens (77,78) and their ability to potentially predict response to neoadjuvant systemic therapies (Tables 4-6) (79-94). For instance, in the NACT portion of the WSG ADAPT trial, Oncotype Dx recurrence scores (RS) performed on presurgical biopsies were predictive of pCR (82). While pCR rates were low overall, patients with an RS >25 had a significantly higher pCR rate than patients with an RS ≤25 (16.1% vs. 7.2%, P=0.006). This difference was most evident amongst premenopausal patients (17.2% vs. 4.8%, P=0.03) while the difference among postmenopausal patients was not significant (15.2% vs. 12.2%, P=0.8). Therefore, if a patient has a preoperative genomic risk score predicting long term benefits from chemotherapy it may be reasonable to administer NACT particularly if tumor or axillary downstaging is required to improve surgical outcomes. Notably, while gene expression profiles may be used in the clinic to guide clinical decision making regarding NACT (95) current guidelines do not recommend their routine use in this setting (15).
Table 4
| Author | Gene expression profile | Study type | Patient population | Number of patients | Treatment | Endpoints | Low risk <11 | Low risk <25 | Intermediate risk 11–25 | High risk >25 | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Morales Murillo et al. 2021 (79) | Oncotype Dx | Prospective | HR pos HER2 neg | 60 | NACT NS | RCB 0/1 | NA | PostMp: 6.7%, PreMp: 0%, RS (11–20) | PostMp: 52.6%, PreMp: 42.9%, RS >20 | NA | |
| Bear et al. 2017 (80) | Oncotype Dx | Prospective | HR pos HER2 neg | 64 | Anthracycline/taxane NACT or ET | CRR, BCS, pCR | ET: 83.3%, 75%, 0% | ET: 50%, 72.2%, 0%, CT: 72.7%, 63.6%, 0% | CT: 92.9%, 57.1%, 14.3% | 0.049, NA, NA | |
| Sella et al. 2021 (81) | Oncotype Dx | Retrospective | HR pos HER2 neg age <40 | 76 | Anthracycline/taxane NACT | pCR | 5% | 21% | 0.09 | ||
| Kuemmel et al. 2020 (82) | Oncotype Dx | Prospective | HR pos HER2 neg | 864 | Anthracycline/taxane NACT | pCR | 7%, PostMp: 12.2%, PreMp: 4.8% | 16%, PostMp: 15.2%, PreMp: 17.2% | 0.006, 0.8, 0.003 | ||
| Thekkekara et al. 2019 (83) | Oncotype Dx | Retrospective | HR pos HER2 neg | 110 | NACT NS | CRR, pCR | 32.5%, 0% | 81.4%, 16% | NA, NA |
RS, recurrence score; HR, hormone receptor; pos, positive; neg, negative; NACT, neoadjuvant chemotherapy; NS, nonsignificant; RCB, residual cancer burden; NA, not available; PostMp, postmenopausal; PreMp, premenopausal; ET, endocrine therapy; CRR, clinical response rate; BCS, breast conserving surgery; pCR, pathological complete response; CT, chemotherapy.
Table 5
| Author | Gene expression profile | Study type | Patient population | Number of patients | Treatment | End points | Low risk <18 | Intermediate risk 18–30 | High risk >30 | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Pardo et al. 2021 (84) | Oncotype Dx | Retrospective | HR pos HER2 neg | 158 | NACT not specified | Axillary pCR | 10.7% | 9.7% | 27.5% | 0.0268 |
| Iwata et al. 2019 (85) | Oncotype Dx | Prospective | HR pos HER2 neg | 295 | Letrozole | CRR, BCS | 54%, 79% | 42%, NA | 22%, 60% | <0.001, 0.009 |
| Pivot et al. 2015 (86) | Oncotype Dx | Prospective | HR pos HER2 neg | 81 | Anthracycline/taxane NACT | pCR | 0% | 6.2% | 8.6% | 0.004 |
| Yardley et al. 2015 (87) | Oncotype Dx | Prospective | HER2 neg | 108 | Ixabepilone/cyclophosphamide | pCR | 0% | 0% | 17% (HR neg) 31% (HR pos) | 0.002 |
| Ueno et al. 2014 (88) | Oncotype Dx | Prospective | HR pos | 64 | Exemestane | CRR | 59.4% | 58.8% | 20% | 0.015 |
| Akashi-Tanaka et al. 2009 (89) | Oncotype Dx | Prospective | HR pos | 43 | Tamoxifen or anastrazole | CRR | 64% | 31% | 31% | 0.11 |
| Chang et al. 2008 (90) | Oncotype Dx | Prospective | Locally advanced all subtypes | 97 | Docetaxel | CRR | 0% | NA | 21.4% | NA |
RS, recurrence score; HR, hormone receptor; pos, positive; neg, negative; NACT, neoadjuvant chemotherapy; pCR, pathological complete response; BCS, breast conserving surgery; CRR, clinical response rate; NA, not available.
Table 6
| Author | Gene expression profile | Study type | Patient population | Number of patients | Treatment | End points | Low risk | Intermediate risk | High risk | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Dubsky et al. 2020 (91) | Endopredict | Retrospective | HR pos HER2 neg | 134 | Anthracycline/taxane NACT ± tecemotide | RCB 0/1 | 0% | NR | 26.4% | 0.112 |
| Dubsky et al. 2020 (91) | Endopredict | Retrospective | HR pos HER2 neg | 83 | Letrozole ± tecemotide | RCB 0/1 | 27.3% | NR | 7.7% | |
| Whitworth et al. 2017 (92) | Mammaprint/Blueprint | Prospective | HR pos HER2 neg | 474 | Anthracycline/taxane NACT | pCR | 2% | NR | 13% | 0.001 |
| Mathieu et al. 2012 (93) | BCI | Retrospective | All subtypes | 150 | Anthracycline/taxane NACT | pCR, BCS | 1.6%, 14% | 21%, 46% | 29%, 44% | 0.0001, 0.0002 |
| Straver et al. 2010 (94) | Mammaprint | Retrospective | All subtypes | 167 | Anthracycline/taxane NACT ± trastuzumab | pCR | 0% | NR | 20% | 0.015 |
HR, hormone receptor; pos, positive; neg, negative; NACT, neoadjuvant chemotherapy; RCB, residual cancer burden; NR, not reported; pCR, pathological complete response; BCI, breast cancer index; BCS, breast conserving surgery.
Neoadjuvant endocrine treatment (NET)
For post-menopausal HR positive patients in need of surgical downstaging who are either not candidates or are not predicted to benefit from chemotherapy, another option is NET. Currently, due to a limited amount of data in premenopausal patients, NET should not be regularly recommended in this patient population. Although pCR is rarely achieved with NET, clinical response rate (CRR) and BCS rates, while varying between trials, appear to be comparable to NACT and with less toxicity (96).
The pivotal trials in NET have demonstrated the superiority of aromatase inhibitors (AIs) over tamoxifen in terms of response rates and surgical outcomes. P024 randomized 337 postmenopausal BCS-ineligible patients to 4 months of NET with letrozole or tamoxifen (97) with superior CRRs (55% vs. 36%, P<0.001) and BCS rates (45% vs. 35%, P=0.022) associated with the letrozole. In PROACT, 451 postmenopausal patients were randomized to 12 weeks of preoperative anastrozole or tamoxifen (98) with concomitant chemotherapy allowed. Among the 262 patients treated with NET alone and ineligible for upfront BCS the CRR was significantly superior with anastrozole (49% vs. 36%, P=0.04). There were no significant differences in BCS between the two groups (38% vs. 30%, P=0.11). PROACT also provided data on axillary downstaging. Amongst the 201 patients with node positive disease, 43.4% of patients in the letrozole group and 38.5% of patients in the tamoxifen group experienced clinical downstaging of the axilla. To date there are limited prospective data regarding the approach to the axilla following NET with retrospective data indicating between a 10–15% axillary pCR rate (99). The IMPACT trial randomized 330 postmenopausal patients to 12 weeks of preoperative anastrozole, tamoxifen or the combination (100). CRRs were similar between groups and amongst the 124 patients initially ineligible for BCS, 44% of those treated with anastrozole had BCS compared with 31% receiving tamoxifen (P=0.23). Additionally, the rate of patients deemed eligible by their surgeons for BCS were significantly higher following anastrozole than tamoxifen or the combination (46%, 22% and 26% respectively, P=0.03). This study also provided early biomarker data as higher levels of ER were shown to correlate with response. Additionally, tumor cell proliferation as measured by a decrease in Ki-67 levels 2 weeks following treatment was significantly improved in the anastrozole group (101) and was associated with improved recurrence free survival (102).
NACT vs. NET
The largest trial comparing NACT to NET randomized 239 postmenopausal women to NET with an AI (exemestane or anastrozole) or to NACT with doxorubicin and paclitaxel (103). CRRs were 64% in both the NET and chemotherapy arms, pCR rates were low in both arms (3% and 6% respectively) and there was a non-statistically significant numerical difference in BCS rates in favor of NET (33% vs. 24%, P=0.058). Kim et al. (104) randomized 187 premenopausal women to anthracycline/taxane based NACT or NET with goserelin and tamoxifen with the primary endpoint of CRR at 24 weeks. While there were no differences in BCS (13.8% vs. 11.5%, P=0.531), patients receiving NACT had a significantly better CRR (84% vs. 71%, P=0.046). In GEICAM/2006-03, 95 patients, 51 of which were premenopausal, were randomized to NET with exemestane (+ goserelin if premenopausal) or NACT (105). Similarly, premenopausal women experienced significantly greater CRR to NACT (75% vs. 44%, P=0.027), while no difference was seen among post-menopausal women (57% vs. 52%, P=0.78). The pCR rates were exceptionally low in both groups (NACT: 2%, NET: 0%) and there were no differences in BCS or axillary nodal status after surgery.
Potential biomarkers of response to NET
With pCR being a rare occurrence, data from the earlier NET trials supported the development of a distinct surrogate pathologic marker of response to NET known as preoperative endocrine prognostic index (PEPI) (106). This score was developed by analyzing post treatment factors associated with survival in P024 and independently validated in a cohort of patients from IMPACT. PEPI is based on the post-NET surgical specimen and calculated as the sum of points given to 4 categories: tumor size, nodal status, Ki-67 level, and ER expression. Patients with a PEPI of 0 (pT0/1, N0, Ki67 <2.7%, and positive ER), have very favorable outcomes without chemotherapy. In ACOSOG Z1031 377 postmenopausal patients were randomized to 16–18 weeks of NET with an AI (letrozole, anastrozole or exemestane) (107) with comparable CRR and BCS rates between arms. The PEPI score was a secondary endpoint and tumors were subtyped by a PAM-50 analysis. CRRs were 62.9%, 74.8% and 69.1% for exemestane, letrozole, and anastrozole, respectively. In patients designated as requiring a mastectomy before treatment 51% were subsequently able to undergo BCS, and 83% of patients who were considered marginal for breast conservation underwent BCS. There was no difference between CRR or BCS rates between luminal A and luminal B cancers, however significantly more patients with luminal A disease had a PEPI score of 0 (27.1% vs. 10.7%, P=0.004). At a median follow-up of 5.5 years, of 421 patients from Z1031 eligible for long-term analysis, 119 (25.9%) had a PEPI 0 response and only 4 (3.3%) recurrences were identified in this group as opposed to 49 (14.4%) in patients with a PEPI score >0 (108).
NET in premenopausal women
As discussed, 2 studies comparing NACT to NET showed a significantly greater CRR in premenopausal patients receiving NACT. The phase 3 STAGE trial randomized 197 premenopausal patients to 24 weeks of preoperative goserelin with anastrozole or tamoxifen (109). Patients in the anastrozole group had a CRR of 70.4% vs. 50.5% in the tamoxifen group (P=0.004). Despite this promising trial, data is still limited on the role of NET in this patient population and more studies are needed to properly identify premenopausal patients who are most likely to benefit from this treatment approach.
The main findings of the major NET trials are summarized in Table 7.
Table 7
| Trial | Year | Treatment arms | Patient population | Number of patients | CRR | BCS rate | P value (CRR, BCS rate) |
|---|---|---|---|---|---|---|---|
| Eiermann et al. (97) | 2001 | Letrozole vs. tamoxifen, 4 months | Post-menopausal, stage II/III, BCS ineligible | 337 | Letrozole =55%, tamoxifen =37% | Letrozole =45%, tamoxifen =35% | <0.001, 0.022 |
| Cataliotti et al. (98) | 2006 | Anastrozole vs. tamoxifen ± CT, 12 weeks | Postmenopausal, tumor size >3 cm | 262 (ET alone) | Anastrozole =49%, tamoxifen =36% | Anastrozole =38%, tamoxifen =30% | 0.04, 0.11 |
| Smith et al. (100) | 2005 | Anastrozole, tamoxifen or both, 3 months | Postmenopausal | 330 | Anastrozole =37%, tamoxifen =36%, combination =39% | Anastrozole =44%, tamoxifen =31% | 0.87, 0.23 |
| Semizaglov et al. (103) | 2007 | A + T vs. exemestane or anastrozole, 3 months | Post-menopausal, stage II/III | 239 | ET =64.5%, CT =63.6% | ET =33%, CT =24% | >0.5, 0.058 |
| Kim et al. (104) | 2020 | AC-T vs. goserelin + tamoxifen, 24 weeks | Pre-menopausal, stage II/III | 187 | 84%, 71% | 13.8%, 11.5% | 0.046, 0.531 |
| Alba et al. (105) | 2012 | AC-T vs. exemestane ± goserelin, 24 weeks | Pre/post-menopausal, stage II/III | 95 | Premenopausal: ET =44%, CT =75%; postmenopausal: ET =52%, CT =57% | ET =56%, CT =47% | 0.78, 0.2369 |
| Ellis et al. (107) | 2011 | Anastrozole, letrozole, exemestane | Postmenopausal, stage II/III | 377 | Anastrozole =69%, letrozole =75%, exemestane =63% | Anastrozole =77%, letrozole =61%, exemestane =68%, 51% BCS ineligible underwent BCS in entire cohort | NA, NA |
| Masuda et al. (109) | 2012 | Goserelin + tamoxifen or anastrozole, 24 weeks | Premenopausal | 197 | Anastrozole =70%, tamoxifen =50% | Anastrozole =86%, tamoxifen =68% | 0.004, NA |
NET, neoadjuvant endocrine treatment; CRR, clinical response rate; BCS, breast conserving surgery; CT, chemotherapy; ET, endocrine therapy; A, doxorubicin; T, taxane; C, cyclophosphamide; NA, not available.
NET combined with CDK4/6 inhibitors
CDK4/6 inhibitors (abemaciclib, palbociclib, ribociclib) in combination with endocrine therapy have become a standard of care in metastatic HR positive breast cancer (110). Their role in the neoadjuvant/adjuvant setting is still under investigation. The NeoPAL study randomized 106 Prosigna defined luminal A or B stage 2 or 3 patients ineligible for BCS to either letrozole plus palbociclib or standard anthracycline and taxane based chemotherapy (111). The pCR rates were low in both arms (two patients in the palbociclib arm and three in the chemotherapy arm) and the CRRs and BCS rates were identical. The single arm NeoPalana trial (n=50) examined whether the addition of palbociclib to anastrozole increased the rate of complete cell cycle arrest (CCCA) defined as Ki67 <2.7% (112). CCCA was observed among 26% of patients following anastrozole as opposed to 87% after combined treatment (112). Similar improvements in CCCA were observed with abemaciclib in the neoMONARCH trial (113). Thus, while current data indicate that the addition of CDK4/6 inhibition may increase the antiproliferative effect of endocrine treatment and dramatically decrease Ki-67 expression, to date no study has shown an improvement in CRR or BCS rates which are the primary goal of NET. The optimal endpoint to neoadjuvant CDK4/6 trials and their effect on long term outcomes is also unclear. In the adjuvant setting, early results from the MonarchE study comparing adjuvant endocrine therapy with an AI with or without 2 years of abemaciclib in high risk patients showed a significant improvement in 2-year iDFS (114). In contrast two adjuvant trials using palbociclib failed to show an improvement in DFS (115,116). While promising, in the neoadjuvant setting these agents should currently only be used within a clinical trial.
Conclusions
Over the last two decades, we have come to understand that neoadjuvant systemic therapy is as safe and effective as adjuvant therapy (2). In patients with operable breast cancer neoadjuvant therapy can be considered for all patients determined upfront to require systemic adjuvant treatment. If given preoperatively this treatment may improve surgical outcomes. In patients with TN and HER-2 positive tumors, neoadjuvant systemic therapy should also be considered not only for the improvement of surgical outcomes, but also for the prognostic and predictive information the response to treatment will provide. Neoadjuvant therapy also offers a window of opportunity to research novel biomarkers allowing for a more tailored approach to patient care. At present, the role of neoadjuvant systemic therapy in early breast cancer in both contemporary clinical practice and the research setting is continuing to develop with the likelihood that its applications will continue to expand, further emphasizing the importance of multidisciplinary communication to provide the best outcomes for our patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard and Jørn Bo Thomsen) for the series “Breast Reconstruction—The True Multidisciplinary Approach” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-109/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-109/coif). The series “Breast Reconstruction—The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. OG declares receiving Honoraria from Eli-Lilly, Roche, and Pfizer, advisory board for Eli-Lilly support for meetings from Pfizer and Medison. ENGY declares receiving honoraria from Eli-Lilly, Roche, Pfizer, Novartis, MSD, and Astra Zeneca, advisory boards for Eli-Lilly, Roche, Pfizer, Novartis, MSD, and AstraZeneca, and support for meetings from Pfizer and Roche. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mougalian SS, Soulos PR, Killelea BK, et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer 2015;121:2544-52. [Crossref] [PubMed]
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997;15:2483-93. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
- Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013;14:609-18. [Crossref] [PubMed]
- Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455-61. [Crossref] [PubMed]
- Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0-T4, N1-N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance). Ann Surg 2016;263:802-7. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- Symmans WF, Wei C, Gould R, et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J Clin Oncol 2017;35:1049-60. [Crossref] [PubMed]
- Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414-22. [Crossref] [PubMed]
- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med 2017;376:2147-59. [Crossref] [PubMed]
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 2019;380:617-28. [Crossref] [PubMed]
- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014;32:3744-52. [Crossref] [PubMed]
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010;375:377-84. [Crossref] [PubMed]
- Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015;372:134-41. [Crossref] [PubMed]
- Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J Clin Oncol 2021;39:1485-505. [Crossref] [PubMed]
- Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res 2007;13:228-33. [Crossref] [PubMed]
- Sikov WM, Dizon DS, Strenger R, et al. Frequent pathologic complete responses in aggressive stages II to III breast cancers with every-4-week carboplatin and weekly paclitaxel with or without trastuzumab: a Brown University Oncology Group Study. J Clin Oncol 2009;27:4693-700. [Crossref] [PubMed]
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol 2014;15:640-7. [Crossref] [PubMed]
- von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 2017;377:122-31. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [Crossref] [PubMed]
- Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278-84. [Crossref] [PubMed]
- van der Voort A, van Ramshorst MS, van Werkhoven ED, et al. Three-Year Follow-up of Neoadjuvant Chemotherapy With or Without Anthracyclines in the Presence of Dual ERBB2 Blockade in Patients With ERBB2-Positive Breast Cancer: A Secondary Analysis of the TRAIN-2 Randomized, Phase 3 Trial. JAMA Oncol 2021;7:978-84. [Crossref] [PubMed]
- O'Shaughnessy J, Robert N, Annavarapu S, et al. Recurrence rates in patients with HER2+ breast cancer who achieved a pathological complete response after neoadjuvant pertuzumab plus trastuzumab followed by adjuvant trastuzumab: a real-world evidence study. Breast Cancer Res Treat 2021;187:903-13. [Crossref] [PubMed]
- Chan A, Moy B, Mansi J, et al. Final Efficacy Results of Neratinib in HER2-positive Hormone Receptor-positive Early-stage Breast Cancer From the Phase III ExteNET Trial. Clin Breast Cancer 2021;21:80-91.e7. [Crossref] [PubMed]
- ECOG-ACRIN Cancer Research Group. CompassHER2-pCR: Preoperative THP and Postoperative HP in Patients Who Achieve a Pathologic Complete Response. Report No. NCT04266249. Available online: https://clinicaltrials.gov/ct2/show/NCT04266249
- Jules Bordet Institute. De-Escalation of Adjuvant Chemotherapy in HER2-positive, Estrogen Receptor-negative, Node-negative Early Breast Cancer Patients Who Achieved Pathological Complete Response After Neoadjuvant Chemotherapy and Dual HER2 Blockade. Report No. NCT04675827. Available online: https://clinicaltrials.gov/ct2/show/NCT04675827
- Hurvitz SA, Martin M, Jung KH, et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study. J Clin Oncol 2019;37:2206-16. [Crossref] [PubMed]
- Nitz UA, Gluz O, Christgen M, et al. De-escalation strategies in HER2-positive early breast cancer (EBC): final analysis of the WSG-ADAPT HER2+/HR- phase II trial: efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab ± weekly paclitaxel. Ann Oncol 2017;28:2768-72. Erratum in: Ann Oncol 2022;33:355. [Crossref] [PubMed]
- Filho OM, Viale G, Stein S, et al. Impact of HER2 Heterogeneity on Treatment Response of Early-Stage HER2-Positive Breast Cancer: Phase II Neoadjuvant Clinical Trial of T-DM1 Combined with Pertuzumab. Cancer Discov 2021;11:2474-87. [Crossref] [PubMed]
- Llombart-Cussac A, Cortés J, Paré L, et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol 2017;18:545-54. [Crossref] [PubMed]
- Trivers KF, Lund MJ, Porter PL, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control 2009;20:1071-82. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432-44. [Crossref] [PubMed]
- Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in Early Breast Cancer: The ABC Trials—USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol 2017;35:2647-55. [Crossref] [PubMed]
- Nitz U, Gluz O, Clemens M, et al. West German Study PlanB Trial: Adjuvant Four Cycles of Epirubicin and Cyclophosphamide Plus Docetaxel Versus Six Cycles of Docetaxel and Cyclophosphamide in HER2-Negative Early Breast Cancer. J Clin Oncol 2019;37:799-808. [Crossref] [PubMed]
- Sharma P, López-Tarruella S, García-Saenz JA, et al. Pathological Response and Survival in Triple-Negative Breast Cancer Following Neoadjuvant Carboplatin plus Docetaxel. Clin Cancer Res 2018;24:5820-9. [Crossref] [PubMed]
- Sharma P, Kimler BF, O'Dea A, et al. Randomized Phase II Trial of Anthracycline-free and Anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative Breast Cancer (NeoSTOP). Clin Cancer Res 2021;27:975-82. [Crossref] [PubMed]
- Bines J, Earl H, Buzaid AC, et al. Anthracyclines and taxanes in the neo/adjuvant treatment of breast cancer: does the sequence matter? Ann Oncol 2014;25:1079-85. [Crossref] [PubMed]
- Sparano JA, Zhao F, Martino S, et al. Long-Term Follow-Up of the E1199 Phase III Trial Evaluating the Role of Taxane and Schedule in Operable Breast Cancer. J Clin Oncol 2015;33:2353-60. [Crossref] [PubMed]
- Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003;21:1431-9. [Crossref] [PubMed]
- Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol 2016;17:345-56. [Crossref] [PubMed]
- Untch M, Jackisch C, Schneeweiss A, et al. NAB-Paclitaxel Improves Disease-Free Survival in Early Breast Cancer: GBG 69-GeparSepto. J Clin Oncol 2019;37:2226-34. [Crossref] [PubMed]
- Gianni L, Mansutti M, Anton A, et al. Comparing Neoadjuvant Nab-paclitaxel vs Paclitaxel Both Followed by Anthracycline Regimens in Women With ERBB2/HER2-Negative Breast Cancer-The Evaluating Treatment With Neoadjuvant Abraxane (ETNA) Trial: A Randomized Phase 3 Clinical Trial. JAMA Oncol 2018;4:302-8. [Crossref] [PubMed]
- Poggio F, Bruzzone M, Ceppi M, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol 2018;29:1497-508. [Crossref] [PubMed]
- von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014;15:747-56. [Crossref] [PubMed]
- Loibl S, Weber KE, Timms KM, et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol 2018;29:2341-7. [Crossref] [PubMed]
- Sikov WM, Polley MY, Twohy E, et al. CALGB (Alliance) 40603: Long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT) +/- carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). J Clin Oncol 2019;37:591. [Crossref]
- Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015;33:13-21. [Crossref] [PubMed]
- Hahnen E, Lederer B, Hauke J, et al. Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer: Secondary Analysis of the GeparSixto Randomized Clinical Trial. JAMA Oncol 2017;3:1378-85. [Crossref] [PubMed]
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol 2017;8:561. [Crossref] [PubMed]
- Zhao T, Li C, Wu Y, et al. Prognostic value of PD-L1 expression in tumor infiltrating immune cells in cancers: A meta-analysis. PLoS One 2017;12:e0176822. [Crossref] [PubMed]
- Disis ML, Stanton SE. Triple-negative breast cancer: immune modulation as the new treatment paradigm. Am Soc Clin Oncol Educ Book 2015;e25-30. [Crossref] [PubMed]
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 2018;379:2108-21. [Crossref] [PubMed]
- Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020;396:1817-28. [Crossref] [PubMed]
- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382:810-21. [Crossref] [PubMed]
- Schmid P, Cortes J, Dent R, et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med 2022;386:556-67. [Crossref] [PubMed]
- Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 2020;396:1090-100. [Crossref] [PubMed]
- Gianni L, Huang C, Egle D, et al. Pathologic complete response to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. San Antonio Breast Cancer Symposium 2019.
- Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 2019;30:1279-88. [Crossref] [PubMed]
- Loibl S, Schneeweiss A, Huober JB, et al. Durvalumab improves long-term outcome in TNBC: results from the phase II randomized GeparNUEVO study investigating neodjuvant durvalumab in addition to an anthracycline/taxane based neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC). J Clin Oncol 2021;39:506. [Crossref]
- Spiers L, Coupe N, Payne M. Toxicities associated with checkpoint inhibitors-an overview. Rheumatology (Oxford) 2019;58:vii7-16. [Crossref] [PubMed]
- Pogoda K, Niwińska A, Sarnowska E, et al. Effects of BRCA Germline Mutations on Triple-Negative Breast Cancer Prognosis. J Oncol 2020;2020:8545643. [Crossref] [PubMed]
- Gonçalves A, Bertucci A, Bertucci F. PARP Inhibitors in the Treatment of Early Breast Cancer: The Step Beyond? Cancers (Basel) 2020;12:1378. [Crossref] [PubMed]
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917-21. [Crossref] [PubMed]
- Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017;355:1152-8. [Crossref] [PubMed]
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018;379:753-63. [Crossref] [PubMed]
- Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017;377:523-33. Erratum in: N Engl J Med 2017;377:1700. [Crossref] [PubMed]
- Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 2019;30:558-66. [Crossref] [PubMed]
- Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med 2021;384:2394-405. [Crossref] [PubMed]
- Rugo HS, Olopade OI, DeMichele A, et al. Adaptive Randomization of Veliparib-Carboplatin Treatment in Breast Cancer. N Engl J Med 2016;375:23-34. [Crossref] [PubMed]
- Geyer CE, Sikov WM, Huober J, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol 2022;33:384-94. [Crossref] [PubMed]
- Litton JK, Scoggins ME, Hess KR, et al. Neoadjuvant Talazoparib for Patients With Operable Breast Cancer With a Germline BRCA Pathogenic Variant. J Clin Oncol 2020;38:388-94. [Crossref] [PubMed]
- Litton JK, Beck JT, Jones JM, et al. Neoadjuvant talazoparib in patients with germline BRCA1/2 (gBRCA1/2) mutation-positive, early HER2-negative breast cancer (BC): Results of a phase 2 study. J Clin Oncol 2021;39:505. [Crossref]
- Sparano JA, Gray RJ, Makower DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 2018;379:111-21. [Crossref] [PubMed]
- Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg 2014;260:608-14; discussion 614-6. [Crossref] [PubMed]
- Sakorafas GH, Peros G, Cataliotti L, et al. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol 2006;15:153-65. [Crossref] [PubMed]
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206-23. [Crossref] [PubMed]
- Qi P, Yang Y, Bai QM, et al. Concordance of the 21-gene assay between core needle biopsy and resection specimens in early breast cancer patients. Breast Cancer Res Treat 2021;186:327-42. [Crossref] [PubMed]
- Jakubowski DM, Bailey H, Abran J, et al. Molecular characterization of breast cancer needle core biopsy specimens by the 21-gene Breast Recurrence Score test. J Surg Oncol 2020;122:611-8. [Crossref] [PubMed]
- Morales Murillo S, Gasol Cudos A, Veas Rodriguez J, et al. Selection of neoadjuvant treatment based on the 21-GENE test results in luminal breast cancer. Breast 2021;56:35-41. [Crossref] [PubMed]
- Bear HD, Wan W, Robidoux A, et al. Using the 21-gene assay from core needle biopsies to choose neoadjuvant therapy for breast cancer: A multicenter trial. J Surg Oncol 2017;115:917-23. Erratum in: J Surg Oncol 2018;118:722; J Surg Oncol 2021;124:914. [Crossref] [PubMed]
- Sella T, Gelber SI, Poorvu PD, et al. Response to neoadjuvant chemotherapy and the 21-gene Breast Recurrence Score test in young women with estrogen receptor-positive early breast cancer. Breast Cancer Res Treat 2021;186:157-65. [Crossref] [PubMed]
- Kuemmel, S. Gluz O, Nitz U et al. Neoadjuvant nab-paciltaxel weekly vs dose-dense paclitaxel followed by dose-dense EC in high risk HR+/HER2- early BC: results from the neoadjuvant part of ADAPT HR+/HER2- trial. San Antonio Breast Cancer Symposium 2020.
- Thekkekara RJ, Bharadwaj S, Yadav U, et al. Predicting response to neoadjuvant chemotherapy in nonmetastatic hormone receptor-positive breast cancer using 21-gene Breast Recurrence Score test. J Clin Oncol 2019;37:e12093. [Crossref]
- Pardo JA, Fan B, Mele A, et al. The Role of Oncotype DX® Recurrence Score in Predicting Axillary Response After Neoadjuvant Chemotherapy in Breast Cancer. Ann Surg Oncol 2021;28:1320-5. [Crossref] [PubMed]
- Iwata H, Masuda N, Yamamoto Y, et al. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study. Breast Cancer Res Treat 2019;173:123-33. [Crossref] [PubMed]
- Pivot X, Mansi L, Chaigneau L, et al. In the era of genomics, should tumor size be reconsidered as a criterion for neoadjuvant chemotherapy? Oncologist 2015;20:344-50. [Crossref] [PubMed]
- Yardley DA, Peacock NW, Shastry M, et al. A phase II trial of ixabepilone and cyclophosphamide as neoadjuvant therapy for patients with HER2-negative breast cancer: correlation of pathologic complete response with the 21-gene recurrence score. Breast Cancer Res Treat 2015;154:299-308. [Crossref] [PubMed]
- Ueno T, Masuda N, Yamanaka T, et al. Evaluating the 21-gene assay Recurrence Score® as a predictor of clinical response to 24 weeks of neoadjuvant exemestane in estrogen receptor-positive breast cancer. Int J Clin Oncol 2014;19:607-13. [Crossref] [PubMed]
- Akashi-Tanaka S, Shimizu C, Ando M, et al. 21-Gene expression profile assay on core needle biopsies predicts responses to neoadjuvant endocrine therapy in breast cancer patients. Breast 2009;18:171-4. [Crossref] [PubMed]
- Chang JC, Makris A, Gutierrez MC, et al. Gene expression patterns in formalin-fixed, paraffin-embedded core biopsies predict docetaxel chemosensitivity in breast cancer patients. Breast Cancer Res Treat 2008;108:233-40. [Crossref] [PubMed]
- Dubsky PC, Singer CF, Egle D, et al. The EndoPredict score predicts response to neoadjuvant chemotherapy and neoendocrine therapy in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer patients from the ABCSG-34 trial. Eur J Cancer 2020;134:99-106. [Crossref] [PubMed]
- Whitworth P, Beitsch P, Mislowsky A, et al. Chemosensitivity and Endocrine Sensitivity in Clinical Luminal Breast Cancer Patients in the Prospective Neoadjuvant Breast Registry Symphony Trial (NBRST) Predicted by Molecular Subtyping. Ann Surg Oncol 2017;24:669-75. [Crossref] [PubMed]
- Mathieu MC, Mazouni C, Kesty NC, et al. Breast Cancer Index predicts pathological complete response and eligibility for breast conserving surgery in breast cancer patients treated with neoadjuvant chemotherapy. Ann Oncol 2012;23:2046-52. [Crossref] [PubMed]
- Straver ME, Glas AM, Hannemann J, et al. The 70-gene signature as a response predictor for neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 2010;119:551-8. [Crossref] [PubMed]
- Pease AM, Riba LA, Gruner RA, et al. Oncotype DX® Recurrence Score as a Predictor of Response to Neoadjuvant Chemotherapy. Ann Surg Oncol 2019;26:366-71. [Crossref] [PubMed]
- Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant Endocrine Therapy for Estrogen Receptor-Positive Breast Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;2:1477-86. [Crossref] [PubMed]
- Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol 2001;12:1527-32. [Crossref] [PubMed]
- Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative "Arimidex" Compared to Tamoxifen (PROACT) trial. Cancer 2006;106:2095-103. [Crossref] [PubMed]
- Stafford A, Williams A, Edmiston K, et al. Axillary Response in Patients Undergoing Neoadjuvant Endocrine Treatment for Node-Positive Breast Cancer: Systematic Literature Review and NCDB Analysis. Ann Surg Oncol 2020;27:4669-77. [Crossref] [PubMed]
- Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108-16. [Crossref] [PubMed]
- Dowsett M, Smith IE, Ebbs SR, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res 2005;11:951s-8s. [Crossref] [PubMed]
- Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 2007;99:167-70. [Crossref] [PubMed]
- Semiglazov VF, Semiglazov VV, Dashyan GA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 2007;110:244-54. [Crossref] [PubMed]
- Kim HJ, Noh WC, Lee ES, et al. Efficacy of neoadjuvant endocrine therapy compared with neoadjuvant chemotherapy in pre-menopausal patients with oestrogen receptor-positive and HER2-negative, lymph node-positive breast cancer. Breast Cancer Res 2020;22:54. [Crossref] [PubMed]
- Alba E, Calvo L, Albanell J, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol 2012;23:3069-74. [Crossref] [PubMed]
- Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380-8. [Crossref] [PubMed]
- Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J Clin Oncol 2011;29:2342-9. [Crossref] [PubMed]
- Ellis MJ, Suman VJ, Hoog J, et al. Ki67 Proliferation Index as a Tool for Chemotherapy Decisions During and After Neoadjuvant Aromatase Inhibitor Treatment of Breast Cancer: Results From the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol 2017;35:1061-9. [Crossref] [PubMed]
- Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol 2012;13:345-52. [Crossref] [PubMed]
- Schettini F, Giudici F, Giuliano M, et al. Overall Survival of CDK4/6-Inhibitor-Based Treatments in Clinically Relevant Subgroups of Metastatic Breast Cancer: Systematic Review and Meta-Analysis. J Natl Cancer Inst 2020;112:1089-97. [Crossref] [PubMed]
- Cottu P, D'Hondt V, Dureau S, et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann Oncol 2018;29:2334-40. [Crossref] [PubMed]
- Ma CX, Gao F, Luo J, et al. NeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast Cancer. Clin Cancer Res 2017;23:4055-65. [Crossref] [PubMed]
- Hurvitz SA, Martin M, Press MF, et al. Potent Cell-Cycle Inhibition and Upregulation of Immune Response with Abemaciclib and Anastrozole in neoMONARCH, Phase II Neoadjuvant Study in HR+/HER2- Breast Cancer. Clin Cancer Res 2020;26:566-80. [Crossref] [PubMed]
- Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol 2020;38:3987-98. [Crossref] [PubMed]
- Mayer EL, Dueck AC, Martin M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2021;22:212-22. [Crossref] [PubMed]
- Loibl S, Marmé F, Martin M, et al. Palbociclib for Residual High-Risk Invasive HR-Positive and HER2-Negative Early Breast Cancer-The Penelope-B Trial. J Clin Oncol 2021;39:1518-30. [Crossref] [PubMed]
Cite this article as: Globus O, Greenhouse I, Sella T, Gal-Yam EN. The neoadjuvant systemic treatment of early breast cancer: a narrative review. Ann Breast Surg 2023;7:39.

