
Can imaging findings predict the outcome of idiopathic granulomatous mastitis?
Introduction
Idiopathic granulomatous mastitis (IGM) is a chronic inflammatory condition that can mimic various benign and malignant conditions of the breast, especially infectious mastitis, and inflammatory breast cancer (1,2). It has an increased incidence in non-white women, especially Asian, Hispanic, African American and Middle Eastern ethnicity (1,3-5). The most common manifestation of IGM is a palpable painful unilateral breast mass followed by isolated skin induration (3-6). In up to 55% of patients with IGM, malignancy is the primary concern based on presentation and radiologic findings (7). Due to significant overlap of findings with breast cancer, tissue biopsy is needed in most cases to establish the correct diagnosis (1,3,6,8-15).
The etiology of IGM remains unclear, however, pregnancy and lactation history are known to be associated with IGM (1,16). Hyperprolactinemia also increases the risk of IGM development (17,18). Although IGM is accepted to be a non-infectious process, an association with infection by Corynebacterium kroppenstedtii, which requires fastidious culture and prolonged antibiotic treatment, has been proposed (19).
There is no standard treatment for IGM (15,20). Treatment options include observation alone with intermittent imaging follow-up, vs. administration of corticosteroid or methotrexate, and in more severe cases wide local excision or mastectomy (1,3,21).
The prognosis of IGM is variable, ranging from a chronic relapsing disease in some patients to complete resolution in others. The variability in the prognosis also depends on the length of follow-up and treatment modality (22); hence, different recurrence and complete resolution rates have been reported (5–25%, 15–80%, respectively) (3,23-27).
Ultrasound (US) and mammography are the primary imaging modalities to establish IGM diagnosis (28). The most common finding on mammography is focal or global asymmetry (36–75%), and on US irregular tubular extensions (40–100%) which is not among the descriptors defined in the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) US lexicon (28,29). Due to the overlap of IGM imaging features with cancer, and lack of well-defined criteria for descriptors and response assessment, the role of imaging in treatment follow-up and surveillance of IGM is unclear. Furthermore, there is no consensus regarding surveillance of IGM to monitor treatment response. It is also unclear whether imaging findings at baseline or improvement at follow-up is associated with final disease outcome.
We hypothesized that the clinical assessment is non-inferior to imaging in determining disease severity or correlation with final prognosis. Therefore, we aimed to investigate the relationship between imaging features of IGM at baseline, as well as improvement at follow-up and disease prognosis, and compare them to clinical assessment performed at same time points. Showing lack of imaging benefit may help obviate unnecessary imaging and benign biopsies in patients with established or suspected IGM diagnosis. We present the following article in accordance with the STROBE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-22-23/rc).
Methods
Patient selection
The study was approved by the Institutional Review Board of The University of Texas Southwestern Medical Center (IRB registration number: IORG0000638) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived as this study is a retrospective review. We reviewed all women with presumed IGM diagnosis at our institution (University of Texas Southwestern Medical Center and Parkland Hospital, Dallas, TX, USA) between April 5, 2010 and December 14, 2017. Patients with biopsy-proven diagnosis and/or clinical diagnosis of IGM were included in our study. For patients diagnosed with bilateral IGM, we collected the timing of involvement (metachronous vs. synchronous) and analysis was performed on a per breast basis.
Data collection
We collected patient age, ethnicity, parity, date of last childbirth, lactation status, menopausal status, biopsy side, biopsy method, pathology result, date of initial US performed and performing site [emergency department, primary care physician (PCP) office or breast clinic], initial US findings, number of weeks between patient’s initial clinical visit and breast imaging, initial BI-RADS assessment, initial US and mammography (if any) findings, first-line treatment modality (treatment modality after breast clinic encounter), if patient unresponsive to first-line treatment second line treatment information (treatment modality after first-follow up visit), date of first follow-up US and findings, clinical status at first follow-up (improved, not improved or no follow-up), results of first follow-up US (improved, not improved or no follow-up), and number of months between the first and last US. Our reference standard was disease prognosis, i.e., final disease outcome. We categorized this as: completely resolved (clinical complete resolution) or unresolved (i.e., either improved with minimal residual symptoms, or stable/worsening disease). Clinical improvement assessment was made based on physicians’ notes on electronic medical record system. Imaging assessment of improvement in disease severity was performed by two fellowship-trained breast radiologists, with 10–21 years of experience in breast imaging, based on lesion size, extent (number of breast quadrants involved) and skin changes. Differential diagnosis of tuberculosis, fungal disease, sarcoidosis, and other rheumatologic diseases, such as rheumatoid arthritis, and vasculitis were considered in our study and patient charts were reviewed for these etiologies. Acid fast staining was ordered in patients with fistula formation or other manifestations of tuberculous breast involvement. All remaining specimens undergo acid-fast staining at the discretion of the pathologist.
Imaging technique
All mammograms were performed in one of the 4 commercially available units (Dimensions, Hologic) at a single institution. Diagnostic mammogram consisted of a combination of full-field digital mammography craniocaudal and mediolateral oblique views and spot compression views.
US examinations were performed and interpreted by 1 of 7 breast imaging radiologists using 1 of 3 iU22 (Philips Healthcare) units with high-frequency transducers (L12-5 and/or L17-5 linear array transducers).
All studies were interpreted by 1 of 7 board-certified, breast imaging fellowship-trained radiologists with 5–20 years of experience independently reviewed at a dedicated workstation (McKesson).
Statistical analysis
Summary statistics of imaging and clinical findings with patient characteristics were provided in frequencies and percentages. In patients whose follow-up imaging was available, we used Fisher exact test and Chi-square test to assess the association of improvement seen on first imaging or first clinical follow-up with the final outcome. To compare treatment vs. observation group disease outcome, we used Chi-Square test. Logistic regression analysis was performed to assess the association between the imaging findings at presentation, extent of involvement, and the final outcome in patients with available outcome information. The Mann-Whitney U test was conducted to compare the time passed until first improvement seen in follow-up imaging and clinical follow-up. A P value of less than 0.05 was statistically meaningful. When a case had missing data for any of the variables, we excluded that case from the analysis. All statistical analysis were performed using IBM SPSS Statistics 28.0.
Results
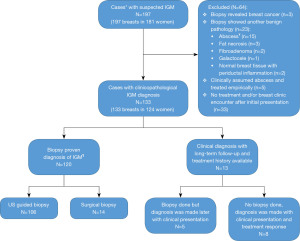
We identified 197 breasts in 181 women with presumed IGM by imaging findings during the study period. Of these, 133/197 (67.5%) were diagnosed either based on clinical presentation and positive response to steroid therapy (n=13, 9.8%) or tissue sampling (n=120, 90.2%). Remaining were excluded due to lack of tissue verification, tissue sampling that revealed a different pathology, or lack of breast clinic encounter after initial presentation at emergency department/PCP office. Excluded cases and reasons for exclusion are illustrated in Figure 1. Bilateral breast involvement was present in 9 women (3 were synchronous, 6 metachronous). Differential diagnosis of tuberculosis, fungal disease, sarcoidosis, and other rheumatologic diseases, such as rheumatoid arthritis, and vasculitis were considered in our study. None of the patients had either a history or clinical/imaging findings to indicate breast involvement of a systemic granulomatous inflammation.
Mean patient age was 35.8 years [standard deviation (SD): ±6.7 years] and 120/124 (96.8%) were premenopausal. Most patients (117/124, 94.4%) were of Hispanic or Latino ethnicity. One hundred and eight (87.1%) had a history of childbirth, 104 (104/108, 96.3%) were >2 years post-partum while 1 (1/124, 0.8%) was lactating at the time of diagnosis.
Of 133 breasts with IGM, complete disease resolution occurred in 44 (33.1%) (categorized as resolved), improvement with subclinical disease in 59 (44.4%), worsening disease in 18 (13.5%), (categorized as unresolved, n=77). In the remaining 12 (9.0%) there was no follow-up imaging, and the final outcome was unknown.
Patient age, ethnicity/race, parity and menopausal status are outlined in Table 1.
Table 1
| Clinical variables | Number of patients (%) |
|---|---|
| Mean age (± SD) (years) | 35.8 (±6.7) |
| Ethnicity/race | |
| Hispanic or Latino | 117 (94.4) |
| African-American | 4 (3.2) |
| Non-Hispanic White | 2 (1.6) |
| Native American | 1 (0.8) |
| Menopausal status | |
| Premenopausal | 120 (96.8) |
| Postmenopausal | 2 (1.6) |
| Unknown | 2 (1.6) |
| Parity | |
| Remote‡ | 104 (83.9) |
| Recent childbirth | 4 (3.2) |
| Pregnant | 10 (8.1) |
| Nulliparous | 6 (4.8) |
| Total | 124 (100.0) |
‡, >2 years passed since childbirth. SD, standard deviation.
Baseline findings on US and mammogram
Initial presentation was to emergency department (80/133, 60.2%), PCP (45/133, 33.8%) and breast clinic (8/133, 6.0%). All patients were seen in the breast clinic and US was performed. Of those 133, 93 (69.9%) also underwent mammography. Median time between patients’ initial encounter at hospital and breast clinic was 4 weeks [interquartile range (IQR), 2–7 weeks]. Overall, the most common finding on US was hypoechoic collection (101/133, 75.9%) with 53.5% (54/101) having associated skin involvement. Focal asymmetry (48/93, 51.6%) was the most common mammographic finding followed by global asymmetry (17/93, 18.3%), and mass (16/93,17.2%). Abnormal lymph node(s) on mammogram were observed in 8/93 (8.6%). Most patients received a BI-RADS Category 4 (76/133, 57.1%) followed by BI-RADS Category 3 (26/133, 19.5%), BI-RADS Category 2 (26/133, 19.5%), and BI-RADS Category 5 (3/133, 2.3%), while mammogram was negative in 2 [BI-RADS category 1, 2/133 (1.5%)]. Imaging findings at presentation are summarized in Table 2.
Table 2
| Biopsy method and imaging findings | Number (%) |
|---|---|
| Laterality | |
| Left | 69 (51.9) |
| Right | 64 (48.1) |
| Biopsy method | |
| Ultrasound guided core biopsy | 111 (83.5) |
| Surgical excision | 14 (10.5) |
| No biopsy | 8 (6.0) |
| Ultrasound findings at ED presentation | |
| Hypoechoic collection only | 24 (18.0) |
| Hypoechoic collection + skin involvement* | 22 (16.5) |
| Hypoechoic mass | 5 (3.8) |
| Skin involvement only | 3 (2.3) |
| No imaging findings | 8 (6.0) |
| No imaging performed in ED¥ | 71 (53.4) |
| Mammogram findings | |
| Focal asymmetry | 48 (36.1) |
| Global asymmetry | 17 (12.8) |
| Mass | 16 (12.0) |
| Nipple retraction | 2 (1.5) |
| Amorphous calcifications | 2 (1.5) |
| Skin thickening | 1 (0.8) |
| No mammographic abnormalities | 7 (5.3) |
| No mammogram | 40 (30.0) |
| Ultrasound findings | |
| Hypoechoic collection | 101 (75.9) |
| Skin involvement* | 54 (40.6) |
| Drainable fluid collection | 26 (19.5) |
| Isolated mass | 27 (20.3) |
| Subareolar involvement | 48 (36.1) |
| Number of quadrants involved | |
| 1 | 103 (77.4) |
| 2 | 22 (16.5) |
| ≥3 | 8 (6.1) |
| Total | 133 (100.0) |
*, skin involvement is defined as skin thickening, fistula formation, and/or intradermal collection; ¥, 53/71 (74.7%) did not present to ED at first encounter. 45/53 (84.9%) were first seen by a primary care physician and 8/53 (15.1%) at breast clinic. ED, emergency department.
First-line treatment, follow-up imaging and second line treatment
Of the 125 breasts which were initially evaluated either in the emergency department (n=80) or by PCP (n=45), 69 (55.2%) were initially treated prior to referral to breast clinic. Of these, 65 (94.2%) received antibiotics and only 4 (5.8%) received steroids. After breast clinic encounter, 131 breasts had available first-line treatment information and of those 131, 56 (42.7%) received steroids, 14 (10.7%) received antibiotics, 7 (5.3%) underwent incision and drainage or surgical excision, and 41 (31.3%) were observed clinically.
Follow-up US was performed in 91/133 (68.4%) breasts and median time to first follow-up US was 4 months (IQR, 2–10 months). US findings were improved in 42/91 (46.2%) breasts vs. not improved in 49/91 (53.8%) breasts. All patients with follow-up US also had clinical follow-up and median time to first follow-up was 4 months (IQR, 2–8 months). Of the 91 patients 63 (69.2%) showed clinical improvement and 28 (30.8%) showed no clinical improvement. Median time between follow-up US and clinical follow-up was 1 week (IQR, 0–6 weeks).
Of the 133 breasts, 130 (97.7%) were also followed in the breast clinic. While 91/130 (70.0%) showed clinical improvement, 39/130 (30.0%) did not improve clinically. In breasts with imaging follow-up, the first improvement in disease findings was identified at a median of 6 months with US (IQR, 2.0–7.5 months) vs. 4 months clinically (IQR, 2.0–10.5 months) (P=0.25).
Second line treatment choice in patients with follow-up US (91/133, 68.4%) is presented in Table S1.
US-guided aspiration was performed in 24 (18.0%) cases. Of these, 3 (12.5%) showed progressive disease course, 1 (4.2%) showed complete resolution and the remaining 20 (83.3%) improved with minimal residual symptoms. Of note, only 1 out of 24 (4.2%) received US-guided aspiration and observation alone (both first and second line) resulting in improvement with minimal residual symptoms.
Overall, the treatment group (n=100) (patients treated with at least one treatment modality, either first or second line) had more US studies performed (median, 3; IQR, 2.0–5.0) than the observation group (n=33) (median, 2; IQR, 1.0–2.5) (P<0.001). No difference was seen in final disease outcome (resolved vs. unresolved) between observation and treatment group (46.2% vs. 33.2% resolved respectively, P=0.24). Similarly, no difference was seen in follow-up US result (improved vs. not improved) between observation and treatment group (61.5% vs. 43.6% respectively, P=0.23).
Association of imaging findings vs. clinical follow-up findings with disease outcome
Time between first breast center US and final disease outcome evaluation varied between patients (median, 12 months; IQR, 2–32 months). We did not find any association between baseline imaging features and final disease outcome (resolved vs. unresolved) (P=0.18–0.93) (Table 3).
Table 3
| Lesion characteristics | Final disease outcome, n (%) | ||
|---|---|---|---|
| Resolved (n=44) | Unresolved (n=77) | P value* | |
| Hypoechoic collection | 0.32 | ||
| Yes | 32 (34.0) | 62 (66.0) | |
| No | 12 (44.4) | 15 (55.6) | |
| Intradermal collection | 0.19 | ||
| Yes | 12 (44.4) | 15 (55.6) | |
| No | 32 (34.0) | 62 (66.0) | |
| Skin involvement† | 0.69 | ||
| Yes | 18 (35.3) | 33 (64.7) | |
| No | 26 (37.1) | 44 (62.9) | |
| Drainable fluid collection | 0.24 | ||
| Yes | 7 (28.0) | 18 (72.0) | |
| No | 37 (38.5) | 59 (61.5) | |
| Mass | 0.78 | ||
| Yes | 10 (47.6) | 11 (52.4) | |
| No | 34 (34.0) | 66 (66.0) | |
| Subareolar involvement | 0.93 | ||
| Yes | 18 (40.0) | 27 (60.0) | |
| No | 26 (34.2) | 50 (65.8) | |
| Fistula to skin | 0.42 | ||
| Yes | 1 (16.7) | 5 (83.3) | |
| No | 43 (37.4) | 72 (62.6) | |
| Number of quadrants involved | 0.18 | ||
| 1 | 1 (33.3) | 2 (66.7) | |
| 2 | 29 (32.2) | 61 (67.8) | |
| ≥3 | 14 (50.0) | 14 (50.0) | |
†, skin involvement is defined as skin thickening, fistula formation, and/or intradermal collection; *, P values were calculated using the Wald test.
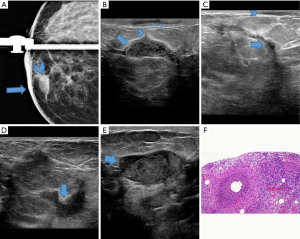
Overall, there was no association between initial improvement in imaging and/or clinical findings at first follow-up (improved vs. not improved) and eventual disease resolution (42.9% vs. 24.5%, P=0.06; 36.5% vs. 25.0%, P=0.28; respectively). In addition, no association was shown in the first 6 months (25.0% vs. 21.7%; P=1.00), 9 months (23.1% vs. 25.9%; P=1.00) and 12 months (26.1% vs. 24.1%; P=0.87) between improvement in imaging and disease outcome. Similarly, clinical improvement at first follow-up was not associated with disease outcome (improved vs. not improved 6 months, 26.3% vs. 23.8%, P=0.83; 9 months, 29.8% vs. 25.0%, P=0.67; 12 months, 30.0% vs. 26.9%, P=0.78) (see Table 4). An example of a refractory case, despite initial improvement on follow-up imaging, is provided in Figure 2.
Table 4
| Follow-up | Final disease outcome, n (%) | ||
|---|---|---|---|
| Resolved | Unresolved | P value | |
| 0–6 months | |||
| Imaging | 1.00* | ||
| Improved | 2 (25.0) | 6 (75.0) | |
| Not improved | 5 (21.7) | 18 (78.3) | |
| Clinical | 0.83** | ||
| Improved | 10 (26.3) | 28 (73.7) | |
| Not improved | 5 (23.8) | 16 (76.2) | |
| 0–9 months | |||
| Imaging | 1.00* | ||
| Improved | 3 (23.1) | 10 (76.9) | |
| Not improved | 7 (25.9) | 20 (74.1) | |
| Clinical | 0.67** | ||
| Improved | 14 (29.8) | 33 (70.2) | |
| Not improved | 6 (25.0) | 18 (75.0) | |
| 0–12 months | |||
| Imaging | 0.87** | ||
| Improved | 6 (26.1) | 17 (73.9) | |
| Not improved | 7 (24.1) | 22 (75.9) | |
| Clinical | 0.78** | ||
| Improved | 15 (30.0) | 35 (70.0) | |
| Not improved | 7 (26.9) | 19 (73.1) | |
| All follow-up | |||
| Imaging | 0.06** | ||
| Improved | 18 (42.9) | 24 (57.1) | |
| Not improved | 12 (24.5) | 37 (75.5) | |
| Clinical | 0.28** | ||
| Improved | 23 (36.5) | 40 (63.5) | |
| Not improved | 7 (25.0) | 21 (75.0) | |
Denominators are variable due to analyzing different time frames. *, P values were calculated using the Fisher exact test; **, P values were calculated using the Chi-Square test.
Discussion
In our study, IGM outcome could not be predicted on follow-up US imaging findings. Our findings indicate that follow-up US imaging in IGM was not helpful to predict the disease outcome, and US follow-up was not superior to clinical follow-up. In our study group, median time to first follow-up US was 4 months (IQR, 2–10 months) with a wide range of 0–68 months consistent with the waxing and waning nature of the disease, which likely contributes to the irregular follow-up in patients with IGM.
Additionally, there was no statistically significant difference in between the time interval between treatment and improvement in imaging findings compared to the time interval between treatment and clinical improvement (median, 6 months; IQR, 2.0–7.5 months vs. median, 4 months; IQR, 2.0–10.5 months, respectively; P=0.25). Because of this, imaging follow-up does not offer any clear advantages over clinical follow-up with respect to assessing treatment response.
Some authors proposed breast exam and annual mammography with US every 3–6 months after the acute episode until complete resolution of clinical symptoms (6,7). This approach yields multiple imaging studies and procedures throughout its clinical course, utilizing resources without clear benefit. Our findings are not consistent with such protocol. In our study, US was routinely ordered in most patients regardless of timing of clinical follow-up and many times prior to clinical evaluation after completion of a course of medical treatment. Due to the protracted disease course, we believe clinical follow-up was more influential in the decision to further treat these patients.
We analyzed the association of baseline US imaging features such as intradermal collection, skin involvement, drainable fluid collection, subareolar involvement and fistula to skin with final disease outcome. Baseline imaging features were not found to be successful in anticipating the disease outcome. To our best knowledge, our study is the first one to investigate this relationship.
IGM has a higher incidence in non-white people, especially Asian, Hispanic, and Middle Eastern ethnicity (1,8,24,30). Some authors suggested that the increased reporting of IGM in developing countries such as Jordan, Turkey, Arabia, China, etc. is due to under diagnosis of tuberculous mastitis in these countries (11,12). Two studies from the United States demonstrated predisposition in Hispanic or Latino patients (9,24), though neither of these studies were able to present clinical evident reason for this susceptibility. Similarly, the majority of our patients (117/124, 94.4%) were Hispanic or Latino, without history of tuberculosis per chart review.
Due to the nonspecific clinical manifestations and imaging findings of IGM, the diagnosis is usually based on the presence of specific histopathologic findings when other etiologies of granulomatous breast disease have been ruled out (1,12,31). In our study, we did not find any remarkable association with other etiologies such as histoplasmosis, sarcoidosis, rheumatoid arthritis or vasculitis. Furthermore, any aspirates obtained from fluid collections in the suspected IGM population from this time period were often sent for routine cultures including acid-fast bacteria and fungal to exclude infections before initiating steroid therapy. No cases of tuberculosis were reported.
Tissue biopsy is needed in many IGM patients to establish the diagnosis and rule out bacterial abscess prior to starting steroid treatment. Additionally, many imaging findings of IGM overlap with those of breast cancer (3,6,25). It is not known if the insult to the breast tissue caused by performing biopsy creates an inflammatory environment and causes a flare in IGM (28). In our study, of 13 (9.8%) breasts diagnosed based on clinical course and treatment response, 5 did receive a biopsy yielding inflammation, inconclusive for IGM therefore delaying the IGM diagnosis. Overall, only 8 (8/133, 6.0%) breasts were not biopsied when diagnosed, and the final outcome in all of them (8/8, 100%) were “unresolved”. The remaining 125 (94.0%) breasts were biopsied, and the final outcome was unresolved in 69 (55.2%), resolved in 44 (35.2%), and unknown in 12 (9.6%). Since only 6.0% (8/133) of our cases were not biopsied, comparing the outcome of the two groups (biopsied vs. not biopsied) was not meaningful in our study.
The treatment of IGM remains controversial. A recent meta-analysis compared effectiveness of different treatment modalities based on observed recurrence rates (22). It was found that combining oral steroid therapy and surgery may result in a lower rate of recurrence. However, both surgery and steroid therapy have their own side effects. Prolonged exposure to corticosteroids predisposes patients to known side effects of the medication such as infectious disease exacerbation, glucose intolerance, weight gain, and hypertension. Repeated needle biopsies, aspirations, and surgeries can lead to cutaneous fistula formation at the needle sites, scarring, and disfigurement. Therefore, at our institution, US-guided aspiration is not used for treatment, but is reserved for symptomatic relief if a large collection was identified by US. In our study, observation vs. corticosteroids as first-line treatment choice was not associated with the final disease resolution. While this was not the primary aim of our study, most of our patients either received oral steroid treatment (52/133, 39.1%) or clinical observation (41/133, 30.8%) while surgical excision was employed only in 5 (3.8%) patients as initial treatment.
Our study has some limitations. Retrospective study design introduces bias. All our analyses were performed on a per breast basis due to presence of different clinical course and/or diagnosis time in contralateral breasts in the same patient. Thirteen (9.8%) cases did not receive biopsy verification of IGM. However, all received steroid therapy and 2 years of follow-up that showed either permanent resolution (5/13, 38.5%) or improvement with minimal residual disease (8/13, 61.5%). Hence, authors felt comfortable including these patients within the data cohort. In fact, our findings suggest that initial imaging features consistent with IGM combined with failure to respond to antibiotics in a young pre-menopausal patient may be sufficient to initiate steroid treatment as second line therapy. The limited number of patients and the lack of follow-up US in some patients decreases the statistical power of our analysis. Furthermore, it is challenging to interpret available data due to IGM’s variable time course and the often relapsing-remitting clinical course of chronic cases. IGM is an uncommon disease. Thus, our single-center data consisting of largely pathologically proven IGM, and available long-term clinical and imaging follow-up is likely a representative of cross section of the disease course. While our study is the first one to investigate the relationship between the outcome of IGM and imaging findings, further studies with larger datasets are needed to better understand this.
Conclusions
Imaging findings at baseline and follow-up do not have a strong correlation with final IGM outcome. Correlation of clinical follow-up findings are non-inferior to imaging, hence clinical follow-up in surveillance is similar to imaging, therefore practitioners should utilize imaging judiciously in a cost-effective manner.
Acknowledgments
Funding: This work was supported by an internal grant by the University of Texas Southwestern, Simmons Cancer Center.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-22-23/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-22-23/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-22-23/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of The University of Texas Southwestern Medical Center (IRB registration number: IORG0000638) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived as this study is a retrospective review.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Al-Khaffaf B, Knox F, Bundred NJ. Idiopathic granulomatous mastitis: a 25-year experience. J Am Coll Surg 2008;206:269-73. [Crossref] [PubMed]
- Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol 1972;58:642-6. [Crossref] [PubMed]
- Aghajanzadeh M, Hassanzadeh R, Alizadeh Sefat S, et al. Granulomatous mastitis: Presentations, diagnosis, treatment and outcome in 206 patients from the north of Iran. Breast 2015;24:456-60. [Crossref] [PubMed]
- Baslaim MM, Khayat HA, Al-Amoudi SA. Idiopathic granulomatous mastitis: a heterogeneous disease with variable clinical presentation. World J Surg 2007;31:1677-81. [Crossref] [PubMed]
- Hovanessian Larsen LJ, Peyvandi B, Klipfel N, et al. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol 2009;193:574-81. [Crossref] [PubMed]
- Gautier N, Lalonde L, Tran-Thanh D, et al. Chronic granulomatous mastitis: Imaging, pathology and management. Eur J Radiol 2013;82:e165-75. [Crossref] [PubMed]
- Lai EC, Chan WC, Ma TK, et al. The role of conservative treatment in idiopathic granulomatous mastitis. Breast J 2005;11:454-6. [Crossref] [PubMed]
- Mohammed S, Statz A, Lacross JS, et al. Granulomatous mastitis: a 10 year experience from a large inner city county hospital. J Surg Res 2013;184:299-303. [Crossref] [PubMed]
- Idiopathic granulomatous mastitis in Hispanic women: Indiana, 2006-2008. MMWR Morb Mortal Wkly Rep 2009: Centers for Disease Control and Prevention (CDC). Contract No.: 47.
- Erhan Y, Veral A, Kara E, et al. A clinicopthologic study of a rare clinical entity mimicking breast carcinoma: idiopathic granulomatous mastitis. Breast 2000;9:52-6. [Crossref] [PubMed]
- Bani-Hani KE, Yaghan RJ, Matalka II, et al. Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies. Breast J 2004;10:318-22. [Crossref] [PubMed]
- Ocal K, Dag A, Turkmenoglu O, et al. Granulomatous mastitis: clinical, pathological features, and management. Breast J 2010;16:176-82. [Crossref] [PubMed]
- Oztekin PS, Durhan G, Nercis Kosar P, et al. Imaging Findings in Patients with Granulomatous Mastitis. Iran J Radiol 2016;13:e33900. [Crossref] [PubMed]
- Cheng J, Du YT, Ding HY. Granulomatous lobular mastitis: a clinicopathologic study of 68 cases. Zhonghua Bing Li Xue Za Zhi 2010;39:678-80. [PubMed]
- Bede K, Valente SA. Idiopathic granulomatous mastitis. Ann Breast Surg 2020;4:24. [Crossref]
- Al-Khawari HA, Al-Manfouhi HA, Madda JP, et al. Radiologic features of granulomatous mastitis. Breast J 2011;17:645-50. [Crossref] [PubMed]
- Nikolaev A, Blake CN, Carlson DL. Association between Hyperprolactinemia and Granulomatous Mastitis. Breast J 2016;22:224-31. [Crossref] [PubMed]
- Lin CH, Hsu CW, Tsao TY, et al. Idiopathic granulomatous mastitis associated with risperidone-induced hyperprolactinemia. Diagn Pathol 2012;7:2. [Crossref] [PubMed]
- Taylor GB, Paviour SD, Musaad S, et al. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathology 2003;35:109-19. [PubMed]
- Godazandeh G, Shojaee L, Alizadeh-Navaei R, et al. Corticosteroids in idiopathic granulomatous mastitis: a systematic review and meta-analysis. Surg Today 2021;51:1897-905. [Crossref] [PubMed]
- Akbulut S, Yilmaz D, Bakir S. Methotrexate in the management of idiopathic granulomatous mastitis: review of 108 published cases and report of four cases. Breast J 2011;17:661-8. [Crossref] [PubMed]
- Lei X, Chen K, Zhu L, et al. Treatments for Idiopathic Granulomatous Mastitis: Systematic Review and Meta-Analysis. Breastfeed Med 2017;12:415-21. [Crossref] [PubMed]
- Azizi A, Prasath V, Canner J, et al. Idiopathic granulomatous mastitis: Management and predictors of recurrence in 474 patients. Breast J 2020;26:1358-62. [Crossref] [PubMed]
- Pandey TS, Mackinnon JC, Bressler L, et al. Idiopathic granulomatous mastitis--a prospective study of 49 women and treatment outcomes with steroid therapy. Breast J 2014;20:258-66. [Crossref] [PubMed]
- Gurleyik G, Aktekin A, Aker F, et al. Medical and surgical treatment of idiopathic granulomatous lobular mastitis: a benign inflammatory disease mimicking invasive carcinoma. J Breast Cancer 2012;15:119-23. [Crossref] [PubMed]
- Yabanoğlu H, Çolakoğlu T, Belli S, et al. A Comparative Study of Conservative vs. Surgical Treatment Protocols for 77 Patients with Idiopathic Granulomatous Mastitis. Breast J 2015;21:363-9. [Crossref] [PubMed]
- Sheybani F, Sarvghad M, Naderi H, et al. Treatment for and clinical characteristics of granulomatous mastitis. Obstet Gynecol 2015;125:801-7. [Crossref] [PubMed]
- Pluguez-Turull CW, Nanyes JE, Quintero CJ, et al. Idiopathic Granulomatous Mastitis: Manifestations at Multimodality Imaging and Pitfalls. Radiographics 2018;38:330-56. [Crossref] [PubMed]
- D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. 5th edition. Reston, VA: American College of Radiology, 2013.
- Centers for Disease Control and Prevention (CDC). Idiopathic granulomatous mastitis in Hispanic women - Indiana, 2006-2008. MMWR Morb Mortal Wkly Rep 2009;58:1317-21. [PubMed]
- Dilaveri CA, Mac Bride MB, Sandhu NP, et al. Breast manifestations of systemic diseases. Int J Womens Health 2012;4:35-43. [Crossref] [PubMed]
Cite this article as: Ozcan BB, Merchant K, Goldberg J, Burns Z, Sahoo S, Compton L, Hayes JC, Dogan BE. Can imaging findings predict the outcome of idiopathic granulomatous mastitis? Ann Breast Surg 2023;7:35.

