
The pros and cons of breast screening in older women
Introduction
Breast screening is attractive to older women as breast cancer incidence increases with age and life expectancy (17 years for women aged 70 years) and quality of life in older women is often good. There is also a trend society as a whole, against ageism with enforced retirement at age cut-offs being outlawed in many countries and age cuts offs for the provision of health interventions also being frowned upon.
Radiologists also find reading screening mammograms in older women (aged >70 years) satisfying. The cancer incidence is high and the breast density is low so cancer detection is easier than in younger women. When screening women over 70 years, the invasive cancer detection rate is over 10/1,000 screens. Benign lesions such as cysts and fibroadenomas rarely grow as hormone replacement therapy (HRT) use in older women is rare so the specificity of screening is also superior to that seen when screening younger women while recall rates are similar (Table 1). Around one in three women aged over 70 years recalled from screening have cancer compared to one in five to one in ten in younger women. Therefore, the harm of false positive recall after screening is less common in older women. False positive recall and overdiagnosis are the major harms caused by breast screening.
Table 1
| Women age | Recall rate | Benign biopsy rate |
|---|---|---|
| 50–70 years | 3.7% | 0.91% |
| >70 years | 4.0% | 0.74% |
The frequency of breast cancer death in the UK increases with age with a peak in numbers of breast cancer deaths occurring between the ages of 70 and 90 years (Office of National Statistics 2017 data). However, the proportion of all deaths that are due to breast cancer decreases with age, peaking at 14% in women aged 40–55 years and dropping to 3% in women aged 80 and 2% in women aged 90 (Office of National Statistics).
Screening of older women: evidence for benefit
It must be remembered that the aim of breast screening is not to detect small cancers but to prevent breast cancer death and to reduce life years lost due to breast cancer. The number of women aged over 70 years in the randomised trials of breast cancer screening was small and the data from combined analysis of the Swedish trials showed a relative risk for breast cancer death in those invited for screen compared to controls not invited for screening was 1.18 [95% confidence interval (CI): 0.71–1.79] (1). There is no evidence from randomised trials that screening reduces breast cancer mortality in older women. It could also be stated that there is little evidence from randomised trials that screening does not reduce breast cancer deaths in this age group as the number screened was small.
Further evidence on the efficacy of screening is available from comparing breast cancer mortality in areas where screening has been introduced early compared to similar areas where screening was introduced later. A Swedish study compared breast cancer mortality by age band in two counties where screening was introduced early (109,000 women) to two counties where screen was introduced 7 years later (77,000 women). The two groups were followed up for 11 years. Mortality reductions were seen in younger women [relative risk (RR): 0.64, 95% CI: 0.43–0.97] for women aged 40–49 years and 0.70 (95% CI: 0.54–0.91) for women aged 50–69 years). No mortality reduction was seen in women aged 70–74 years (RR: 1.08, 95% CI: 0.58–2.03) (2).
Overdiagnosis when screening older women
One of the major harms of breast screening is overdiagnosis which is the detection of cancers which would never present clinically and threaten life. The over diagnosis rate in women screened aged 50–70 years is around 10% (3). There are a number of reasons why screening women aged over 70 years would lead to a higher overdiagnosis rate than that found when screening younger women including decreased life expectancy, the presence of more indolent cancers and less masking by breast density leading to a greater lead time.
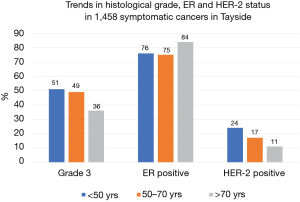
Figure 1 shows the trends in histological grade, ER and HER-2 status in symptomatic breast cancer by age band. The proportion of cancers which are grade 3 decreases with increasing age as does the proportion which are HER-2 positive. The proportion of cancers which are ER positive increases with age. This means the proportion of women dying of causes other than of breast cancer increases with age (Figure 2).
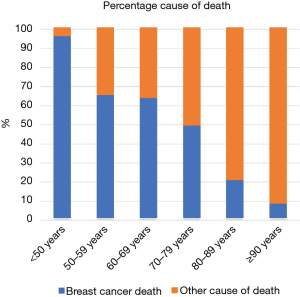
Sixty-five percent of deaths in women aged 50–69 years (the normal screening age group) within 5 years of a symptomatic breast cancer diagnosis are due to metastatic breast cancer. This percentage drops to 20% for women diagnosed in their 80’s and 8% for women diagnosed in their 90’s. This means that the number of symptomatic breast cancers deaths in older women which screening might help avoid is small. The numbers of life years saved by each breast cancer death avoided will also be small.
It should also be noted that tomosynthesis is now commonly used for screening in Europe and the USA, either alone or in combination with full field digital mammography. The use of tomosynthesis is associated with increased cancer detection rates (4). However, the extra cancers detected by tomosynthesis tend to be low grade as tomosynthesis’ main impact is to increase detection of small spiculated masses which are usually low grade. The introduction of tomosynthesis screening has not been associated with significant reductions in interval cancer rates. This suggests that the use of tomosynthesis for screening will lead to an increase in overdiagnosis. Such an increase in overdiagnosis will particularly impact older women attending for screening.
The Impact of overdiagnosis on older women
It is therefore clear that a higher proportion of screen detected cancers in women aged over 70 years represent overdiagnosis compared to women aged 50–69 years. Does the detection and treatment of in-situ and small invasive cancers impact on the quality of life of older women? A recent study has compared changes in health-related quality of life (HRQOL) measured by either the Medical Outcomes Study 36-Item Short Form (SF-36) or the Veterans Rand 12-item Health Survey (VR-12). They compared HRQOL in 198 older women diagnosed with in situ or invasive breast cancer measuring ≤1 cm with 36,814 age matched controls from the SEER cancer registry linked with the Medicare Health Outcomes Survey. The mean age of cases and controls was 75 years. On multivariable analysis, diagnosis of a small breast cancer was found to be one of the strongest predictors of a significant decrease in both the physical and mental domains of HRQOL (P=0.012 and P=0.023, respectively). They concluded that receiving the diagnosis of even a very small breast cancer significantly impacts the physical and mental domains of HRQOL in older women (5). Why is this the case? It might be explained by a recent study from the USA finding many older women did not understand the concept of over-detection and that they were resistant to and suspicious of the concept of over-detection. The authors suggested that providing older women with descriptions of over-detection may not influence screening intentions much.
Attitudes to screening in older women
A report of outcomes of community juries from Australia found that preventive programmes such as mammography screening are likely to have significant symbolic value once they are socially embedded and that arguments for programme de-implementation emphasising declining benefit because of limited life expectancy and the risks of overdiagnosis seem unlikely to resonate with healthy older women. Decisions regarding screening older women are therefore best taken at set-up as withdrawing services once they are established may be difficult. It should however be noted the UK cervical screening has recently stopped screening a young cohort with little adverse reaction from the community.
The UK ageX trial
Scepticism regarding the benefit of screening older women after the UK government announced the extension of the UK screening programme to women aged 71–73 years led to the UK ageX (extension) trial (6). Rather than all women aged 71–73 years being offered screening, half of this age group are invited to screening while half act as a control group and are not invited. Randomisation is by general practitioner practice and not by individual. However, the number of practices within the trial is so large that bias from group randomisation is thought to be very unlikely. The end point is breast cancer mortality. Approximately 2 million women aged over 70 years have been included in the trial. Recruitment began in 2009 and ended in 2020 while the first mortality results are due in 2026. The trial has been extensively criticised for the lack of informed consent of both the study and control groups. However the ageX trial is by far the largest study of screening in this age group and it should provide definitive evidence regarding the harms and benefits of screening women in this age group. If screening is shown to be effective in women over 70 years, additional work will need to be done to identify groups most likely to benefit vs. those most likely to be harmed.
Conclusions
There is currently no conclusive evidence of a reduction in breast cancer mortality when screening women aged over 70 years. Definitive evidence should come from the AgeX trial which is due to report in 2026. Screening women aged over 70 years is associated with less false positive recalls from screening than in younger women. However, overdiagnosis is more common when screening older women than when screening women under 70 years.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kwok-Leung Cheung) for the series “Diagnosis and Treatment on Primary Breast Cancer in Older Women” published in Annals of Breast Surgery. The article has undergone external peer review.
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-114/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at: https://abs.amegroups.com/article/view/10.21037/abs-21-114/coif). The series “Diagnosis and Treatment on Primary Breast Cancer in Older Women” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nyström L, Andersson I, Bjurstam N, et al. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet 2002;359:909-19. [Crossref] [PubMed]
- Jonsson H, Bordás P, Wallin H, et al. Service screening with mammography in Northern Sweden: effects on breast cancer mortality - an update. J Med Screen 2007;14:87-93. [Crossref] [PubMed]
- Zackrisson S, Andersson I, Janzon L, et al. Rate of over-diagnosis of breast cancer 15 years after end of Malmö mammographic screening trial: follow-up study. BMJ 2006;332:689-92. [Crossref] [PubMed]
- Skaane P, Bandos AI, Gullien R, et al. Prospective trial comparing full-field digital mammography (FFDM) versus combined FFDM and tomosynthesis in a population-based screening programme using independent double reading with arbitration. Eur Radiol 2013;23:2061-71. [Crossref] [PubMed]
- Euhus DM, Addae JK, Snyder CF, et al. Change in health-related quality of life in older women after diagnosis of a small breast cancer. Cancer 2019;125:1807-14. [Crossref] [PubMed]
- Moser K, Sellars S, Wheaton M, et al. Extending the age range for breast screening in England: pilot study to assess the feasibility and acceptability of randomization. J Med Screen 2011;18:96-102. [Crossref] [PubMed]
Cite this article as: Evans A. The pros and cons of breast screening in older women. Ann Breast Surg 2024;8:10.

