
Lymphovenous bypass
Introduction
Lymphedema is a chronic, progressive disease characterized by the accumulation of lymphatic fluid in the body due to impaired transport in the lymphatic system. Initially, symptoms include reversible soft tissue swelling from proteinaceous lymphatic fluid collecting in subcutaneous tissues. Over time, symptoms progress to irreversible swelling, increased inflammation, and deposition of fibrofatty tissue. Patients with lymphedema suffer from decreased mobility and function, recurrent infections, loss of body confidence due to disfigurement, emotional and psychological distress, and lower quality of life (1).
In developed countries including the Unites States, the most common cause of lymphedema is secondary to oncologic treatment. A 2013 meta-analysis found that an estimated 21% of breast cancer survivors develop upper extremity lymphedema (2), and this has been associated with risk factors including axillary lymph node dissection, adjuvant regional lymph node radiation therapy, and higher body mass index (BMI) (1-3).
Nonsurgical or conservative treatment is generally considered first line for management of lymphedema. Complete decongestive therapy (CDT) is a multimodal approach typically directed by a trained physical therapist and includes manual lymphatic drainage, daily bandaging, exercise, weight loss if indicated, and meticulous skin and nail care to minimize the risk of infection. However, CDT is expensive and time consuming for patients, with some reporting over 40 hours per week devoted to therapy (4). In addition, it only addresses symptoms and not the underlying cause of lymphedema.
Although there is currently no cure for lymphedema, there has been growing interest in surgical options to help alleviate symptoms. These can be subdivided into physiologic versus debulking surgeries. Physiologic surgeries include lymphovenous bypass (LVB) and vascularized lymph node transfer (VLNT). LVB involves the anastomosis of subdermal lymphatic channels with adjacent venules using supermicrosurgical techniques, helping to drain the excess lymphatic fluid trapped in soft tissues (5). Compared to VLNT, LVB is sometimes considered a more attractive option because it is less invasive and carries minimal risk. However, it can only be performed when functional lymphatic channels are available. VLNT refers to the free tissue transfer of lymph nodes from donor sites such as the supraclavicular, thoracic, mesenteric (omentum), or groin lymph nodes. Though the exact mechanism is unclear, it is thought that the transplanted lymph nodes may induce lymphangiogenesis and/or act as a “sponge” to absorb and redirect excess lymphatic fluid into the vascular system (6). Debulking surgeries include liposuction and direct excision. While these surgeries have been shown to successfully reduce limb volume (7), they are more suited for advanced lymphedema, when limb swelling is caused by the accumulation of fibrofatty tissue rather than lymphatic fluid.
The purpose of this article is to review the history, mechanism of action, surgical considerations, current outcomes data, and future directions of LVB specifically for risk reduction of breast cancer related lymphedema.
Historical background and evolution of technique
The history of LVB is closely linked to the history of microsurgery and has relied on the advent of new surgical techniques, instruments, and imaging modalities (8). The concept of treating extremity lymphedema by “anastomosis of terminal blocked lymphatics to neighboring venules” was first described in the early 1960s by Jacobson and Suarez (9). In the 1980s, as the field of microsurgery developed further, several clinical studies on the successful use of LVB to treat lymphedema were reported (10-14). However, LVB was not popularized until 2000, when Koshima et al. reported using supermicrosurgical techniques to perform LVB for treatment of upper extremity lymphedema (15).
Although the term “LVB” historically represented all procedures physiologically connecting the lymphatic and venous systems, today it most commonly refers to supermicrosurgical anastomosis of lymphatic channels to venules. Other physiologic procedures used to divert congested lymphatic fluid into venous circulation include lymph node to venous anastomosis (LNVA), where a lymph node is transected and the capsule of the node is sutured to the vein wall (16). Though these procedures showed experimental promise, they did not translate to clinical success. This article specifically focuses on supermicrosurgical LVB, and refers to such procedures as simply “LVB”.
The term “supermicrosurgery” was first used by Koshima in 1997 (17,18). It is formally defined as a technique of dissection and anastomosis of vessels <0.8 mm in diameter, and it requires highly delicate microsurgical instruments and sutures with needles <30–80 µm in size (17). As most lymphatic channels are <0.5 mm, successful LVB was not consistently possible until the development of specialized equipment and techniques for supermicrosurgery.
The popularization of LVB also relied on advances in imaging modalities and contrast agents to identify functional lymphatic channels in real time for surgical planning. Older imaging modalities, such as direct lymphangiography and Technetium-99 (Tc-99) radionuclide lymphoscintigraphy, are not suitable for operative guidance as they have poor resolution, provide static images, and are time consuming (6).
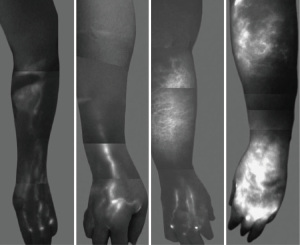
Indocyanine green (ICG) lymphography, which was introduced by Ogata et al. in 2007, is currently the preferred modality for real time visualization of lymphatic channels (19,20). ICG, a non-toxic medical dye with fluorescent properties, is injected intradermally in the web spaces of the affected extremity. Using a near-infrared camera and a laser light source to activate the fluorescence of ICG, lymphatic channels as small as 0.1 mm in diameter can be seen within minutes, identifying suitable channels for LVB as well as the severity of lymphedema based on pattern and dermal backflow (Figure 1). ICG lymphography has also been shown to be more sensitive than lymphoscintigraphy in diagnosing early lymphedema (21). The use of intra-operative ICG lymphography is essential to performing LVB.
Mechanism of action
LVB has been shown to treat lymphedema by mechanically improving lymphatic drainage and by physiologically decreasing the inflammatory changes involved in lymphedema (22). Lymphatic drainage normally occurs via extrinsic and intrinsic forces, with skeletal muscle contraction acting as an extrinsic pump and smooth muscle cells in the walls of lymphatic channels acting as an intrinsic pump (23). LVB creates a connection between the lymphatic and venous systems, bypassing diseased segments of lymphatic channels and acting as lymphovenous shunts. Patients with chronic lymphedema have dilated lymphatic channels and increased lymphatic pressure, which helps with drainage through newly created LVBs as fluid from the higher pressure lymphatic system flows into the lower pressure venous system. However, as lymphedema progresses, the endothelial and smooth muscle cells in the walls of lymphatic channels are destroyed, leading to occlusion particularly at the more proximal aspect of the affected extremity (24). Therefore, LVB is generally performed using the distal lymphatic vessels, which have preserved vessel architecture and therefore a functional intrinsic pump to actively move lymphatic fluid into the recipient vein (24).
In addition to mechanically shunting lymphatic fluid away from diseased channels, LVB has also been shown to attenuate the inflammatory process in lymphedema. The pathophysiology of lymphedema in mouse models involves the activation and proliferation of CD4 T helper 2 (Th2) cells, which promote the production of profibrotic cytokines and growth factors such as transforming growth factor-beta 1 (TGF-β1), interleukin-4 (IL-4), and interleukin-13 (IL-13). Inhibition of these pathways prevents the development of fibrosis, and the severity of lymphedema is positively correlated with the degree of CD4 inflammation (25-27). In 2015, Torrisi et al. demonstrated histological evidence of decreased local tissue CD4 cell inflammation, collagen deposition, hyperkeratosis, and epidermal proliferation in skin biopsies of patients with breast cancer related lymphedema 6 months after LVB (28). Their findings suggest that LVB can potentially reverse some of the pathologic tissue changes seen in lymphedema.
Surgical considerations
Pre-operative workup begins with a detailed history and physical examination, particularly focusing on the etiology, duration of symptoms, and degree of severity of lymphedema. Imaging such as lymphoscintigraphy may be helpful in establishing a diagnosis. There are few contraindications to LVB, except for severe lymphedema where functional lymphatic channels may not be available. In addition, there are fewer risks with LVB compared to VLNT, such as no potential donor site morbidity.
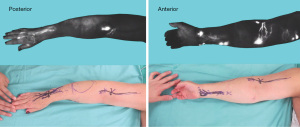
Several surgical techniques for LVB have been described. As with any surgical technique, the principles remain the same, though specific details may vary due to institutional practice and surgeon preference. For the purposes of this review article, we describe the senior author’s current practice. The procedure begins with ICG lymphography to identify lymphatic channels that are potential targets for bypass. 0.01 to 0.02 mL of ICG (Akorn Inc., Lake Forest, IL, USA) is injected intradermally into the web spaces of the lymphedematous extremity. Then, a near-infrared fluorescence imaging system (Photodynamic Eye, Hamamatsu Photonics, Hamamatsu, Japan) is used to visualize patent lymphatic channels, guiding the location of incision sites for LVB (Figure 2) (5). If no potential targets are identified with ICG, it is possible to proceed with LVB by making incisions at defined points and searching for suitable lymphatic channels in these areas (29,30). However, this method is less ideal as it cannot distinguish functional lymphatic channels with an intact intrinsic pump mechanism, which is important for successful outcomes. 0.1 to 0.2 mL of isosulfan blue dye (Lymphazurin, United States Surgical Corp., Norwalk, CT, USA) is also injected in the web spaces to help visually identify lymphatic channels intra-operatively.
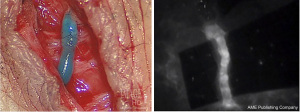
Once a suitable lymphatic channel is found, LVB is performed under the microscope using 11-0 or 12-0 suture to anastomose it to a nearby recipient venule. The recipient venule is tested to ensure there is minimal venous backflow. The anastomosis is usually done in end-to-end fashion if the vessels are similarly sized or end-to-side if the recipient venule is significantly larger or has significant backflow. The patency of the LVB can be confirmed in real time by seeing the isosulfan blue and/or ICG dye travel from the lymphatic channel into the venule (Figure 3). Other anastomotic techniques have been reported in the literature, including various configurations such as “ladder-shaped” multiple side-to-side LVBs (31), pi (π) shaped anastomosis (32), lambda (λ) shaped anastomosis (33), and an “octopus” technique where multiple lymphatic channels are intussuscepted into a single recipient venule (34).
LVB relies not only on the creation of an anastomosis between the lymphatic and venous systems, but also on the maintenance of its patency. Higher venous pressures and excess blood contact have been associated with thrombosis and fibrosis of LVB, so modifications such as distal ligation, proximal valvuloplasty, and creation of pseudovalves to prevent venous stasis and backflow have been described to improve long term patency rates (14,35,36). Some authors also advocate for the use of intravascular stents to help ensure patency (33,37). Meticulous surgical technique has also been shown to be essential to anastomotic success (35). Long term patency rates of LVB in breast cancer related lymphedema are estimated to be at least 56.5% (38).
Post-operatively, patients are seen immediately by the inpatient physical therapy team for compression wrapping. Afterwards they may be discharged, as LVB alone is outpatient surgery. Patients are then instructed to resume previous compression therapy and garments 1 month after surgery to allow the bypasses to mature.
Outcomes
This section highlights published studies in the current literature supporting the efficacy of LVB in treating lymphedema. For a more complete overview, several systematic reviews have demonstrated that LVB results in both quantitative and qualitative improvement in breast cancer related lymphedema (39-42), including a 2021 meta-analysis reporting grade 1C evidence that LVB is effective in reducing the severity of lymphedema (41).
Quantitative improvement
Multiple studies have shown that LVB results in significant limb volume reduction, limb circumference reduction, and decreased episodes of cellulitis. Most of these studies are case series or prospective cohort studies, and many do not stratify results based on the etiology of lymphedema. However, the majority focus on or include upper extremity lymphedema secondary to breast cancer, as this remains the most common cause of secondary lymphedema.
One of the earliest contemporary studies to examine LVB in upper extremity lymphedema was from Koshima et al., who reported a case series showing an average decrease in limb circumference of 4.1 cm (47.3% of pre-operative excess) in 12 patients who underwent LVB and post-operative continuous compression therapy compared to an average decrease in limb circumference of 0.8 cm (11.7% of pre-operative excess) in 12 patients who received compression therapy alone (15). In 2010, Chang et al. reported a prospective study of 20 patients with breast cancer related lymphedema (29). Thirteen out of 20 patients (65%) had quantitative improvement with a mean volume differential reduction of 29% at 1 month, 36% at 3 months, 39% at 6 months, and 35% at 1 year (29). Three years later, the same group reported a prospective study of 89 consecutive cases of LVB for upper extremity lymphedema secondary to breast cancer and found quantitative improvement in 74% of patients (5). The mean volume differential reduction was 33% at 3 months, 36% at 6 months, and 42% at 12 months after LVB, and the reduction was significantly larger in early stage lymphedema (5). Poumellec et al. reported decreased arm circumference after LVB in 31 females with upper extremity lymphedema secondary to breast cancer, with greater improvement seen in early stage lymphedema as shown by 29.5% reduction in stage 2, 13.1% reduction in stage 3, and 0% reduction in stage 4 lymphedema (43). Winters et al. reported a series of 29 patients with breast cancer related lymphedema who underwent LVB, with a preoperative mean difference in arm volumes of 701±435 mL (36.9%), which was reduced to 496±302 mL (24.7%, P=0.00) at 6 months and 467±303 mL (23.5%, P=0.02) at 12 months follow up (44).
In addition to limb size improvement, LVB has also been shown to reduce the frequency of cellulitis in lymphedema. Mihara et al. reported a retrospective review of 95 patients with upper and lower extremity lymphedema who had an average of 1.46 episodes of cellulitis in the year before LVB compared with 0.18 episodes in the year after LVB (P<0.001) (45). Similarly, Pereira et al. reported a mean of 1.3 cellulitis episodes per year pre-operatively compared to 0 cellulitis episodes per year after LVB for upper extremity lymphedema (46).
LVB has also been shown to be a safe procedure with a minimal risk profile. A 2021 systematic review of LVB for upper extremity lymphedema found that no surgical complications were reported except for one episode of skin irritation at the site of contrast injection and one episode of hypertrophic scarring (42).
Qualitative improvement
LVB has also been shown to provide lymphedema patients with symptomatic relief and improved quality of life. Older studies mainly used subjective patient reports, while more recent studies have used validated quality of life measures. Examples of these validated tools include the lymphedema quality of life questionnaire (LYMQOL) which covers 4 domains including function, body image, symptoms, and mood, as well as the Lymphedema International Classification of Functioning questionnaire (Lymph-ICF) which covers 5 domains including physical function, mental function, household activities, mobility, and life and social activities.
Winters et al. found that the overall perceived LYMQOL score increased from 5.8±1.1 to 7.4±0.7 (P=0.00), with significant improvement in each domain (44). Salgarello et al. reported a prospective study of 74 patients with upper or lower extremity lymphedema who underwent LVB with an average follow-up time of 8.5 months. Upper extremity lymphedema patients had an increase of 2.3 points in their overall LYMQOL score, with statistically significant improvement in all 4 domains (47). Cornelissen et al. also reported a prospective study of 20 women with early stage breast cancer related lymphedema who had statistically significant improvement in Lymph-ICF score and all domains 1 year after LVB (48).
A secondary outcome of quality of life reported in some studies is discontinuation of compression therapy. Because compression therapy is typically recommended as part of life-long non-surgical management of lymphedema, the ability to decrease or completely stop wearing compression garments is an outcome linked to improved quality of life. However, there are currently only a few studies which report this outcome, as many surgeons recommend postoperative continuation of compression therapy for a variety of reasons. Such reasons include controlling variables other than LVB in order to determine the efficacy of LVB alone. Of these studies, Winters et al. and Cornelissen et al. reported that 53% and 85% of their patients discontinued compression therapy after LVB, respectively (44,48).
Future directions
While it is generally accepted that LVB is effective in providing both quantitative and qualitative improvement in lymphedema, several topics remain controversial and require further study (49).
Surgical techniques and tools
As previously mentioned in the Surgical Considerations section, there is great variability in LVB technique, including the number of anastomoses. Earlier studies including Koshima’s 2000 study reported better outcomes with higher number of anastomoses (15). However, later studies have shown similar efficacy of LVB regardless of number of anastomoses, including a review of 18 articles by Onoda et al. on LVB for upper and lower extremity lymphedema which concluded that the number of anastomoses did not correlate with effectiveness of LVB (50). However, these findings may be confounded by lymphedema stage, lymphedema etiology, and surgeon experience. The location and configuration of anastomoses may also affect outcomes.
Recent studies have examined the use of nanofibrillar collagen scaffolds in physiologic lymphedema surgery, as they have been shown to guide lymphangiogenesis in porcine models (51). BioBridge™ (Fibralign Corporation, Union City, California) is an implantable surgical mesh ribbon made of a thin membrane with aligned fibrils of purified type 1 porcine collagen (52). Pilot studies have shown improved edema reduction when BioBridge™ implantation is combined with LVB or VLNT (52,53), and more studies are currently ongoing. The use of other biomaterials or growth factors as adjuncts to surgery are potential areas of future investigation.
Current LVB techniques are dependent on imaging technology, as mentioned in the Historical Background section. ICG lymphography is currently used intra-operatively to identify lymphatic channels as potential LVB targets but has several significant limitations. It can only visualize superficial lymphatic channels within 1.5 to 2 cm of the skin surface, cannot detect lymphatic channels when there is dermal backflow, and also does not provide information about the surrounding vasculature (54). Other imaging modalities such as magnetic resonance lymphangiography (MRL) may be more sensitive at detecting the lymphatic system, which may help with pre-operative planning (55,56). Visualization of the deeper lymphatic system with MRL may facilitate LVB within that system, or may highlight connections between the superficial and deep lymphatic systems which have been implicated in distal limb lymphedema such as the hand (57). Additionally, MRL provides other information such as the fibrofatty composition and vascular status of a lymphedematous limb.
Recently, the use of ultrasound is gaining popularity for imaging and aiding LVB. Ultrasound can be used pre-operatively or intra-operatively to identify potential lymphatic channels as well as nearby superficial venules. A comparative study evaluating the correlation between pre-operative imaging findings to the actual lymphatic vessel used for LVB found that high frequency color doppler ultrasound (HFCDU) had significantly higher sensitivity compared to MRL and ICG lymphography (99% compared to 83.5% and 82.3%, respectively), as well as higher specificity and positive predictive value (58). This study also found that HFCDU provided the best detection of lymphatic vessels in more severe stages of lymphedema (58). Though this imaging modality is operator dependent with a steep learning curve, it represents exciting new developments to help facilitate and expand the use of LVB.
Combination surgeries
Another highly debated topic is if and how LVB should be combined with other lymphedema surgeries, both physiologic and debulking. The senior author advocates for performing VLNT and LVB together when possible, as these physiologic interventions work by different mechanisms. A recent study examining the physical and functional outcomes of simultaneous VLNT and LVB found significant improvement in both limb volume reduction and Lymphedema Life Impact Scale (LLIS) post-operatively (59). Interestingly, there was an initial period of improvement which was attributed to LVB, then a “rebound period” 6 months to 1 year post-operatively, followed by gradual improvement which was attributed to VLNT (59).
Since physiologic surgeries do not address the fibrofatty deposition seen in advanced lymphedema, some authors have begun to study combining debulking and physiologic surgeries (60). However, the best patient selection and timing of surgeries is still unknown.
Expanding indications
LVB has traditionally been used for treatment of limb lymphedema. Preventative measures such as prophylactic LVB, also called lymphatic microsurgical preventive healing approach (LYMPHA), are currently being explored with early promising results (61). A more detailed review of LYMPHA is included in a separate article as part of this series on breast cancer related lymphedema.
LVB may also be useful in the treatment of truncal lymphedema. Scaglioni et al. published a case report and literature review of LVB to successfully treat breast lymphedema secondary to oncologic treatment (62).
Conclusions
LVB is a physiologic surgical option for lymphedema treatment. The creation of peripheral connections between the lymphatic and venous systems helps mechanically drain excess lymphatic fluid and physiologically decreases the inflammatory process involved in lymphedema progression. Clinical outcomes show quantitative and qualitative improvement after LVB. Many questions remain about the future directions of LVB to continue optimizing outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Breast Surgery for the series “Breast Cancer-related Lymphedema”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-23-6/coif). The series “Breast Cancer-related Lymphedema” was commissioned by the editorial office without any funding or sponsorship. D.W.C. served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patients for publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Merchant SJ, Chen SL. Prevention and management of lymphedema after breast cancer treatment. Breast J 2015;21:276-84. [Crossref] [PubMed]
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500-15. [Crossref] [PubMed]
- Gillespie TC, Sayegh HE, Brunelle CL, et al. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surg 2018;7:379-403. [Crossref] [PubMed]
- Agarwal S, Garza RM, Chang DW. Lymphatic Microsurgical Preventive Healing Approach (LYMPHA) for the prevention of secondary lymphedema. Breast J 2020;26:721-4. [Crossref] [PubMed]
- Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 2013;132:1305-14. [Crossref] [PubMed]
- Chang DW, Masia J, Garza R 3rd, et al. Lymphedema: Surgical and Medical Therapy. Plast Reconstr Surg 2016;138:209S-18S. [Crossref] [PubMed]
- Boyages J, Kastanias K, Koelmeyer LA, et al. Liposuction for Advanced Lymphedema: A Multidisciplinary Approach for Complete Reduction of Arm and Leg Swelling. Ann Surg Oncol 2015;22:S1263-70. [Crossref] [PubMed]
- Tamai S. History of microsurgery. Plast Reconstr Surg 2009;124:e282-94. [Crossref] [PubMed]
- Jacobson JH 2nd, Suarez EL. Microvascular surgery. Dis Chest 1962;41:220-4. [Crossref] [PubMed]
- O'Brien BM, Sykes P, Threlfall GN, et al. Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg 1977;60:197-211. [Crossref] [PubMed]
- Gong-Kang H, Ru-Qi H, Zong-Zhao L, et al. Microlymphaticovenous anastomosis for treating lymphedema of the extremities and external genitalia. J Microsurg 1981;3:32-9. [Crossref] [PubMed]
- Huang GK, Hu RQ, Liu ZZ, et al. Microlymphaticovenous anastomosis in the treatment of lower limb obstructive lymphedema: analysis of 91 cases. Plast Reconstr Surg 1985;76:671-85. [Crossref] [PubMed]
- O'Brien BM, Mellow CG, Khazanchi RK, et al. Long-term results after microlymphaticovenous anastomoses for the treatment of obstructive lymphedema. Plast Reconstr Surg 1990;85:562-72. [Crossref] [PubMed]
- Gloviczki P, Hollier LH, Nora FE, et al. The natural history of microsurgical lymphovenous anastomoses: an experimental study. J Vasc Surg 1986;4:148-56. [Crossref] [PubMed]
- Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg 2000;16:437-42. [Crossref] [PubMed]
- Calnan JS, Reis ND, Rivero OR, et al. The natural history of lymph node-to-vein anastomoses. Br J Plast Surg 1967;20:134-45. [Crossref] [PubMed]
- Koshima I, Yamamoto T, Narushima M, et al. Perforator flaps and supermicrosurgery. Clin Plast Surg 2010;37:683-9. vii-iii. [Crossref] [PubMed]
- Badash I, Gould DJ, Patel KM. Supermicrosurgery: History, Applications, Training and the Future. Front Surg 2018;5:23. [Crossref] [PubMed]
- Ogata F, Azuma R, Kikuchi M, et al. Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann Plast Surg 2007;58:652-5. [Crossref] [PubMed]
- Ogata F, Narushima M, Mihara M, et al. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann Plast Surg 2007;59:180-4. [Crossref] [PubMed]
- Mihara M, Hara H, Araki J, et al. Indocyanine green (ICG) lymphography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLoS One 2012;7:e38182. [Crossref] [PubMed]
- Garza RM, Chang DW. Lymphovenous bypass for the treatment of lymphedema. J Surg Oncol 2018;118:743-9. [Crossref] [PubMed]
- von der Weid PY, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol 2004;36:1147-53. [Crossref] [PubMed]
- Koshima I, Kawada S, Moriguchi T, et al. Ultrastructural observations of lymphatic vessels in lymphedema in human extremities. Plast Reconstr Surg 1996;97:397-405; discussion 406-7. [Crossref] [PubMed]
- Avraham T, Daluvoy S, Zampell J, et al. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol 2010;177:3202-14. [Crossref] [PubMed]
- Zampell JC, Yan A, Elhadad S, et al. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS One 2012;7:e49940. [Crossref] [PubMed]
- Avraham T, Zampell JC, Yan A, et al. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J 2013;27:1114-26. [Crossref] [PubMed]
- Torrisi JS, Joseph WJ, Ghanta S, et al. Lymphaticovenous bypass decreases pathologic skin changes in upper extremity breast cancer-related lymphedema. Lymphat Res Biol 2015;13:46-53. [Crossref] [PubMed]
- Chang DW. Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plast Reconstr Surg 2010;126:752-8. [Crossref] [PubMed]
- Maegawa J, Mikami T, Yamamoto Y, et al. Types of lymphoscintigraphy and indications for lymphaticovenous anastomosis. Microsurgery 2010;30:437-42. [Crossref] [PubMed]
- Yamamoto T, Kikuchi K, Yoshimatsu H, et al. Ladder-shaped lymphaticovenular anastomosis using multiple side-to-side lymphatic anastomoses for a leg lymphedema patient. Microsurgery 2014;34:404-8. [Crossref] [PubMed]
- Ayestaray B, Bekara F. π-shaped lymphaticovenular anastomosis: the venous flow sparing technique for the treatment of peripheral lymphedema. J Reconstr Microsurg 2014;30:551-60. [Crossref] [PubMed]
- Yamamoto T, Narushima M, Kikuchi K, et al. Lambda-shaped anastomosis with intravascular stenting method for safe and effective lymphaticovenular anastomosis. Plast Reconstr Surg 2011;127:1987-92. [Crossref] [PubMed]
- Chen WF, Yamamoto T, Fisher M, et al. The "Octopus" Lymphaticovenular Anastomosis: Evolving Beyond the Standard Supermicrosurgical Technique. J Reconstr Microsurg 2015;31:450-7. [Crossref] [PubMed]
- Baxter TJ, Gilbert A, O'Brien BM, et al. The histopathology of microlymphaticovenous anastomosis. Aust N Z J Surg 1980;50:320-8. [Crossref] [PubMed]
- Kinjo O, Kusaba A. Lymphatic vessel-to-isolated-vein anastomosis for secondary lymphedema in a canine model. Surg Today 1995;25:633-9. [Crossref] [PubMed]
- Narushima M, Mihara M, Yamamoto Y, et al. The intravascular stenting method for treatment of extremity lymphedema with multiconfiguration lymphaticovenous anastomoses. Plast Reconstr Surg 2010;125:935-43. [Crossref] [PubMed]
- Winters H, Tielemans HJP, Verhulst AC, et al. The Long-term Patency of Lymphaticovenular Anastomosis in Breast Cancer-Related Lymphedema. Ann Plast Surg 2019;82:196-200. [Crossref] [PubMed]
- Scaglioni MF, Fontein DBY, Arvanitakis M, et al. Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery 2017;37:947-53. [Crossref] [PubMed]
- Gasteratos K, Morsi-Yeroyannis A, Vlachopoulos NC, et al. Microsurgical techniques in the treatment of breast cancer-related lymphedema: a systematic review of efficacy and patient outcomes. Breast Cancer 2021;28:1002-15. [Crossref] [PubMed]
- Chang DW, Dayan J, Greene AK, et al. Surgical Treatment of Lymphedema: A Systematic Review and Meta-Analysis of Controlled Trials. Results of a Consensus Conference. Plast Reconstr Surg 2021;147:975-93. [Crossref] [PubMed]
- Gupta N, Verhey EM, Torres-Guzman RA, et al. Outcomes of Lymphovenous Anastomosis for Upper Extremity Lymphedema: A Systematic Review. Plast Reconstr Surg Glob Open 2021;9:e3770. [Crossref] [PubMed]
- Poumellec MA, Foissac R, Cegarra-Escolano M, et al. Surgical treatment of secondary lymphedema of the upper limb by stepped microsurgical lymphaticovenous anastomoses. Breast Cancer Res Treat 2017;162:219-24. [Crossref] [PubMed]
- Winters H, Tielemans HJP, Hameeteman M, et al. The efficacy of lymphaticovenular anastomosis in breast cancer-related lymphedema. Breast Cancer Res Treat 2017;165:321-7. [Crossref] [PubMed]
- Mihara M, Hara H, Furniss D, et al. Lymphaticovenular anastomosis to prevent cellulitis associated with lymphoedema. Br J Surg 2014;101:1391-6. [Crossref] [PubMed]
- Pereira N, Lee YH, Suh Y, et al. Cumulative Experience in Lymphovenous Anastomosis for Lymphedema Treatment: The Learning Curve Effect on the Overall Outcome. J Reconstr Microsurg 2018;34:735-41. [Crossref] [PubMed]
- Salgarello M, Mangialardi ML, Pino V, et al. A Prospective Evaluation of Health-Related Quality of Life following Lymphaticovenular Anastomosis for Upper and Lower Extremities Lymphedema. J Reconstr Microsurg 2018;34:701-7. [Crossref] [PubMed]
- Cornelissen AJM, Kool M, Lopez Penha TR, et al. Lymphatico-venous anastomosis as treatment for breast cancer-related lymphedema: a prospective study on quality of life. Breast Cancer Res Treat 2017;163:281-6. [Crossref] [PubMed]
- Hanson SE, Chang EI, Schaverien MV, et al. Controversies in Surgical Management of Lymphedema. Plast Reconstr Surg Glob Open 2020;8:e2671. [Crossref] [PubMed]
- Onoda S, Satake T, Kinoshita M. Relationship Between Lymphaticovenular Anastomosis Outcomes and the Number and Types of Anastomoses. J Surg Res 2022;269:103-9. [Crossref] [PubMed]
- Hadamitzky C, Zaitseva TS, Bazalova-Carter M, et al. Aligned nanofibrillar collagen scaffolds - Guiding lymphangiogenesis for treatment of acquired lymphedema. Biomaterials 2016;102:259-67. [Crossref] [PubMed]
- Rochlin DH, Inchauste S, Zelones J, et al. The role of adjunct nanofibrillar collagen scaffold implantation in the surgical management of secondary lymphedema: Review of the literature and summary of initial pilot studies. J Surg Oncol 2020;121:121-8. [Crossref] [PubMed]
- Nguyen DH, Zhou A, Posternak V, et al. Nanofibrillar Collagen Scaffold Enhances Edema Reduction and Formation of New Lymphatic Collectors after Lymphedema Surgery. Plast Reconstr Surg 2021;148:1382-93. [Crossref] [PubMed]
- Beederman M, Chang DW. Advances in surgical treatment of lymphedema. Arch Plast Surg 2021;48:670-7. [Crossref] [PubMed]
- Forte AJ, Boczar D, Huayllani MT, et al. Use of magnetic resonance imaging lymphangiography for preoperative planning in lymphedema surgery: A systematic review. Microsurgery 2021;41:384-90. [Crossref] [PubMed]
- Sheng L, Zhang G, Li S, et al. Magnetic Resonance Lymphography of Lymphatic Vessels in Upper Extremity With Breast Cancer-Related Lymphedema. Ann Plast Surg 2020;84:100-5. [Crossref] [PubMed]
- Abdelfattah U, Jaimez PM, Clavero JA, et al. Correlation between superficial and deep lymphatic systems using magnetic resonance lymphangiography in breast cancer-related lymphedema: Clinical implications. J Plast Reconstr Aesthet Surg 2020;73:1018-24. [Crossref] [PubMed]
- Kim HB, Jung SS, Cho MJ, et al. Comparative Analysis of Preoperative High Frequency Color Doppler Ultrasound versus MR Lymphangiography versus ICG Lymphography of Lymphatic Vessels in Lymphovenous Anastomosis. J Reconstr Microsurg 2023;39:92-101. [Crossref] [PubMed]
- Garza RM, Beederman M, Chang DW. Physical and Functional Outcomes of Simultaneous Vascularized Lymph Node Transplant and Lymphovenous Bypass in the Treatment of Lymphedema. Plast Reconstr Surg 2022;150:169-80. [Crossref] [PubMed]
- Brazio PS, Nguyen DH. Combined Liposuction and Physiologic Treatment Achieves Durable Limb Volume Normalization in Class II-III Lymphedema: A Treatment Algorithm to Optimize Outcomes. Ann Plast Surg 2021;86:S384-9. [Crossref] [PubMed]
- Boccardo F, Casabona F, De Cian F, et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: over 4 years follow-up. Microsurgery 2014;34:421-4. [Crossref] [PubMed]
- Scaglioni MF, Meroni M, Fritsche E. Lymphovenous anastomosis (LVA) for treatment of secondary breast lymphedema: A case report and literature review. Microsurgery 2021;41:165-9. [Crossref] [PubMed]
Cite this article as: Huang A, Chang DW. Lymphovenous bypass. Ann Breast Surg 2024;8:6.

