
Contemporary mastectomy options for male breast cancer: nipple-sparing and areolar-sparing mastectomy—a case series
Highlight box
Key findings
• Nipple-sparing mastectomy (NSM) and areolar-sparing mastectomy (ASM) are feasible and safe for select male breast cancer (MBC) patients.
What is known and what is new?
• The majority of MBC is treated with total mastectomy (TM) with removal of the nipple-areolar complex, and many are dissatisfied with the post-mastectomy appearance.
• The MBC patients treated with NSM and ASM in this case series all had negative surgical margins with negative nodal staging and did not require radiation. No postoperative complications or cancer recurrences were observed at median follow-up of 46 months.
What is the implication, and what should change now?
• Contemporary, cosmesis-oriented surgical techniques such as NSM and ASM should be considered as alternatives to TM for select men with breast cancer.
Introduction
Male breast cancer (MBC) is a rare malignancy representing 1% of all breast cancers diagnosed (1). Due to the low incidence of disease and lack of clinical trials involving men, management of MBC is usually extrapolated from treatment of female breast cancer.
In the last three decades, significant surgical advances in women have been focused on breast conserving surgery (BCS), and skin-sparing mastectomy (SSM) or nipple-sparing mastectomy (NSM) with immediate breast reconstruction. NSM which preserves the nipple-areolar complex (NAC) and entire skin envelope has been shown to provide comparable oncologic outcomes as SSM, and is offered for improved cosmesis to appropriately selected women in both the prophylactic and therapeutic setting (2,3). Areolar-sparing mastectomy (ASM) which combines nipple excision with preservation of all or part of the native areola can be performed for tumors involving the nipple or sub-nipple ducts with minimal or no areolar skin involvement (4,5). Preservation of the nipple in women has been associated with improved satisfaction with breasts, sexual and psychosocial wellbeing (6,7). Yet similar surgical options, NSM or ASM, are rarely described for MBC.
Despite a growing body of evidence supporting BCS in men including the National Comprehensive Cancer Network (NCCN), the vast majority of MBC patients are treated with total mastectomy (TM) with NAC removal (8-13). The use of subcutaneous mastectomy or NSM has been reported rarely (14-17). Reconstruction, if performed, is often used to assist with primary closure in advanced cases (10). The predominant use of TM for MBC is likely due to longstanding surgical tradition and the fact that many tumors are primarily centrally located. Other reasons for TM may be perceptions that mastectomy without reconstruction is less disfiguring for a man and less of a cosmetic concern than in women. However, like women, MBC patients experience cancer-related distress and feel embarrassed or self-conscious about the TM scar (18). In the largest MBC patient experience study, many men reported dissatisfaction with their appearance after TM due to loss of the nipple (19). These findings underscore the importance of cosmesis-oriented procedures in the treatment of MBC.
Our case series describes the use of NSM or ASM in select men with early-stage breast cancer. We discuss surgical indications and provide an algorithm for the implementation of NSM or ASMs. We present this article in accordance with the AME Case Series reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-23-64/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee (No. #49549) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
With IRB approval, four patients treated with NSM or ASM by two breast surgeons (M.K., I.W.) at Stanford University Hospital between 2015–2021 were retrospectively identified. Patient and tumor characteristics, treatments, and outcomes are summarized in Table 1. Disease-free interval was defined as no local, regional, or systemic recurrences from time of surgery. Negative margins were defined as no tumor on ink for invasive disease and 2 mm for ductal carcinoma in situ (DCIS).
Table 1
| Variables | Patient A | Patient B | Patient C | Patient D |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (year) | 52 | 66 | 64 | 47 |
| BMI (kg/m2) | 27.3 | 27.6 | 32.4 | 27.5 |
| Genetic mutation | Declined | BRCA2 | None | ATM |
| Tumor characteristics | ||||
| Clinical presentation | Palpable mass | Palpable mass | Palpable mass, nipple discharge | Palpable mass |
| Clinical tumor size (mm) | 22 | 32 | 18 | 13 |
| Distance to nipple on preop imaging (cm) | 1.3 | 1.8 | 0.2 | 1 |
| Histology | IDC | IDC | IDP + ADH (core biopsy); IDC + DCIS (excision) | IDC |
| Grade | 2 | 3 | 2 | 3 |
| Biomarkers | ER+, PR+, HER2+ | ER+, PR+, HER2− | ER+, PR+, HER2− | ER+, PR+, HER2+ |
| Pathologic IDC size (mm) | 24 | 28 | 2 (3 cm DCIS) | 0 (post neo-adjuvant) |
| Pathologic nodal status | N0 | N0 | N0 | N0 |
| Sub-nipple biopsy for cancer | N/A | Positive | Negative | Negative |
| Treatment | ||||
| Surgical procedure | ASM | ASM | NSM | NSM |
| Surgical incision | Radial ellipse | Radial ellipse | Periareolar with radial extension | Inframammary |
| Systemic therapy | Chemotherapy with Herceptin, tamoxifen | Chemotherapy (Oncotype Dx 32), tamoxifen, letrozole | Tamoxifen | Neoadjuvant chemotherapy plus Herceptin and adjuvant tamoxifen |
| Outcomes | ||||
| Ischemia/necrosis | None | None | None | None |
| Disease-free interval (months) | 21 | 67 | 25 | 91 |
ASM, areolar-sparing mastectomy; NSM, nipple-sparing mastectomy; MBC, male breast cancer; BMI, body mass index; IDC, invasive ductal carcinoma; IDP, intraductal papilloma; ADH, atypical ductal hyperplasia; DCIS, ductal carcinoma in situ; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; N/A, not applicable (patient A had nipple removed with the mastectomy).
The median age of the cohort was 58 years, and all presented with a palpable mass, ranging in size from 13 to 32 mm. All had hormone receptor-positive IDC, and two overexpressed HER2. In the three patients treated with upfront surgery (patients A-C), the pathologic invasive tumor size ranged from 2 mm to 2.8 cm with minimal DCIS. Patient C underwent excisional biopsy first for a diagnosis of intraductal papilloma with atypia which led to finding a 2-mm invasive carcinoma and 3 cm of DCIS.
ASM was performed in patients A and B. Patient B was converted from planned NSM to ASM, due to a positive sub-nipple margin biopsy. NSM was performed in Patients C and D. Patient D received neoadjuvant chemotherapy plus anti-HER2 therapy and experienced a pathologic complete response. None required conversion to TM. Sentinel nodes were negative for carcinoma in all patients.
On final pathology, all patients achieved negative surgical margins. No postoperative skin ischemia, necrosis or other complications occurred. No patient required post-mastectomy radiation therapy. On a median follow up of 46 months (range, 21–91 months), no locoregional or distant relapses occurred.
Representative case details
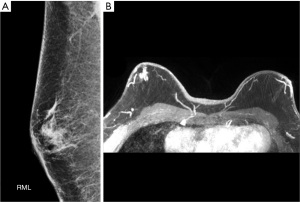
Patient A, a 52-year-old Middle Eastern male, presented with a palpable 3 cm left subareolar mass at 3 o’clock, which measured 1.9 cm on mammogram and 2.2 cm on ultrasound. By magnetic resonance imaging (MRI), the mass measured 2.1 and 1.3 cm from nipple (Figure 1). Core needle biopsy showed IDC, grade 2, estrogen receptor positive (ER+), progesterone receptor positive (PR+), HER2 equivocal on immunohistochemistry but amplified on fluorescence in situ hybridization (FISH). Neoadjuvant chemotherapy was discussed, but patient elected upfront surgery. He underwent partial ASM, nipple reconstruction and sentinel node biopsy (SNB) which showed a 2.4-cm cancer with negative margins and negative nodes (Figure 2). The nipple and some areola overlying the tumor was excised due to close proximity of the tumor for margins. Adjuvant systemic therapy included paclitaxel, trastuzumab, and tamoxifen. He declined genetic testing. At 1.5 years postop, patient reported satisfactory cosmetic outcomes with good nipple projection. Figure 3 demonstrates the 4-year postoperative ASM result in patient B (Figure 3A).



Patient C, a 64-year-old white male, presented with right nipple discharge with a 1.8-cm mass at 10:00 extending from the areolar border into the upper outer quadrant. On mammogram, the mass contained 2.4 cm of calcifications. Breast MRI showed a 1.9 cm area of non-mass enhancement extending to 2 mm from the nipple (Figure 4). Core needle biopsy showed intraductal papilloma with focal atypical ductal hyperplasia (ADH). Surgical excision including removal of the subareolar ducts demonstrated 2 mm IDC, grade 2, ER+, PR+ and HER2−, and 3 cm DCIS with negative margins. Patient wished to forgo radiotherapy and underwent NSM with SNB which contained no residual cancer and negative nodes. He is currently on tamoxifen. Genetic testing was negative. As shown in the postoperative photos at 2 years (Figure 3B), patient was satisfied with the cosmetic appearance despite some volume loss at the scar.
Surgical technique
NSM consists of removal of the breast tissue with preservation of the entire breast skin envelope and NAC (4,20,21). Either a radial or inframammary incision was used (17). A sub-nipple biopsy removing the ducts directly under the nipple was performed to assess for occult carcinoma (5,20,21).
ASM was performed through either a radial or a radially-oriented elliptical incision. An elliptical incision was used if the skin overlying the cancer needed to be excised for adequate margins (Figure 2A). The incision was extended medially directly around the base of the nipple for nipple excision (Figure 2B). The remaining areola is closed by first suturing the areolar edges together and then closing the remaining skin with additional subdermal sutures (Figure 2D). This technique creates an intentional “dog ear” in the middle of the pigmented areolar skin to achieve protuberance and appearance of a nipple or “pseudo-nipple” (Figure 2E).
Discussion
This is the first multi-case series of NSM and ASM for MBC in the literature. It demonstrates the feasibility and safety of NSM or ASM. Although one patient converted from planned NSM to ASM due to a sub-nipple biopsy positive for cancer, none required conversion to TM. There were no postoperative ischemic or necrosis complications. No disease recurrences were observed at a median follow-up of 46 months. This report also describes a novel technique of ASM in which, after excision of the nipple and the areola over the cancer via a radially-oriented ellipse, the remaining areola is reapproximated to create a “pseudo-nipple”.
We hereby propose a contemporary surgical algorithm for MBC incorporating BCS, NSM and ASM (Figure 5). Men with a large breast to tumor ratio should be considered for BCS. In most cases, adjuvant breast irradiation will be recommended, although omission of radiation has been shown to be safe in some women over the age of 70 (13). Breast irradiation causes alopecia, which may cause more noticeable visible changes compared to mastectomy in men. Mastectomies may be better suited for men with a relatively small breast. NSM can be considered for tumors that do not involve the NAC. If there is gross or microscopic disease in the nipple or nipple margin but sparing all or most of the areolar skin, ASM can be considered.

In the modern era of breast cancer treatment, oncologic outcomes are largely driven by tumor biology, and less so by the type of operation. Despite most MBC being biologically favorable and presenting as early stage (9,22), the vast majority are treated with TM, with reported BCS rates ranging from 4% to 23.7% (8-10,22,23). Multiple database analyses and a recent meta-analysis have shown comparable survival between BCS and mastectomy for MBC (8,12,23). A National Cancer Database (NCDB) analysis of 8,445 MBC patients even showed superior survival with BCS and radiation compared to TM with or without radiation (11). Hence, BCS in MBC is feasible and oncologically safe. In fact, the NCCN guidelines state that “decisions about breast conservation versus mastectomy in males should be made according to similar criteria as for females (13)”. The NSM and ASM options presented here represent additional alternatives for MBC, similar to those being offered to female patients.
Prior reports of male NSM in the literature are rare (14-17). In one case, a 54-year-old man underwent bilateral subcutaneous NSM for gynecomastia, and ADH with low grade DCIS was incidentally found in both breasts (16). Removal of the NAC was recommended, but the patient wished to preserve the nipples and was recurrence-free at 18 months. Another report presented a 43-year-old man with clear nipple discharge, mammographic architectural distortion with calcifications sparing the nipple; needle biopsy showed ADH (15). Subcutaneous NSM revealed a single focus of DCIS, and no recurrence was seen at 6 months. The third case was a 44-year-old man with palpable invasive cancer sparing the nipple (14). During the NSM, the nipple margin was benign on frozen section, and intraoperative radiotherapy to the NAC was performed. Patient D in our case series was also previously reported (17). There have been no prior reports in the literature to our knowledge of male ASM.
The term “subcutaneous” mastectomy generally implies that some breast tissue is left behind, particularly under the NAC, and is therefore oncologically not acceptable. In men, subcutaneous mastectomy is usually reserved for symptomatic gynecomastia. The NSMs reported herein were performed similarly to women, by removing all the nipple ducts from the base of the nipple (5,20,21). The oncologic safety of NSM in women has been well established with less than 1% recurrence in the NAC in modern series (24-26). NSM consensus guidelines have been provided by the international Oncoplastic Breast Consortium (OPBC) and NCCN (13,27): (I) NSM can be performed for any tumor size that does not involve the skin or the NAC directly; (II) contraindications to nipple preservation are clinical evidence of nipple involvement and carcinoma at the nipple margin. These same criteria were applied to MBC in this series. Hence, patients with close tumor-to-nipple distance on preoperative imaging can undergo NSM with the above criteria met.
Nipple margin assessment is important, as microscopic disease can be found in the nipple even when there is no clinical or radiographic evidence of nipple involvement. We agree with others and advocate treating the nipple as just another margin (5,27). The risk of occult disease in the nipple may be increased with close tumor-to-nipple distance, large tumor size, and extensive DCIS (28,29). A negative nipple margin carries a 96% negative predictive value, which suggests the NAC can be safely preserved even in high-risk patients (28). A positive nipple margin on sub-nipple biopsy is treated with excision of the nipple, as in patient B in this series, although other management options such as margin re-excision, radiotherapy or observation have been described (5,30). Data for ASM suggest that this approach is safe, if a negative margin can be achieved by nipple excision alone (5,30,31). Preservation of the areola in men provides good cosmesis and symmetry, since nipple height and diameter in men are much less prominent than in women.
Similar to women, men with breast cancer experience cancer-related distress, and altered body image can lead to depression and worsen psychologic distress (32). Many feel embarrassed and self-conscious about a TM scar which serves a “permanent stigma” that they feel necessary to conceal (18). In the largest MBC patient experience study by Chichura et al., all but one patient had a mastectomy, and 61 out of 63 males had nipple removal (19). Thirty-three percent felt uncomfortable with their appearance. Common themes of dissatisfaction were having a “caved or indented” chest, and concerns about scars or lack of nipple. Hence, more cosmesis-oriented procedures such as BCS and NSM or ASM may improve quality of life for men, particularly given our knowledge about improved aesthetic outcomes and psychosocial wellbeing in women undergoing NSM.
Genetic testing is recommended for MBC patients (13). The result may factor into the decision for mastectomy or BCS, as described in our algorithm (Figure 5). Approximately 5–20% of MBC are hereditary, with BRCA2 mutations being the most common. While a BRCA mutation significantly increases a man’s breast cancer risk, the absolute risk is relatively low compared to female BRCA mutation-carriers. Bilateral breast cancer in males is uncommon; therefore, bilateral mastectomies or contralateral prophylactic mastectomy are not routinely recommended for MBC (33,34).
Complication rates after MBC surgery are low. According to NSQIP data, 30-day postoperative morbidity rate was 4.6%, and wound complication rate 3.2%, comparable to many other types of surgery (10). A systematic review of female NSM reported partial or complete nipple necrosis rate of 15%, and other studies indicate a similar range of post-operative ischemic complications (2,35). In our study, male NSM and ASM appear to be surgically safe, but larger series are needed to determine whether ischemic complication rates in NSM are impacted by the smaller skin envelope in men.
This is a small and limited retrospective series that enabled us to review the criteria used to implement these nipple and areolar-sparing mastectomies. The low incidence of MBC precludes a meaningful single institution analysis of oncologic safety. However, it is reasonable to infer that our approach should not be less effective than the same operation in small-breasted women. We hope to better assess oncologic outcomes of NSM and ASM in MBC patients via multi-institutional collaborations. More prospective registries such as the International Male Breast Cancer Program should be opened to evaluate various surgical outcomes for MBC (9).
Conclusions
In this case series, we highlight the feasibility of NSM and ASM for MBC, and delineate a surgical algorithm for these contemporary surgical options. We advocate applying the same surgical criteria for female breast cancer to MBC and offering select MBC patients these cosmesis-oriented procedures as alternatives to TM.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-23-64/rc
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-23-64/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-23-64/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee (No. #49549) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giordano SH. Breast Cancer in Men. N Engl J Med 2018;378:2311-20. [Crossref] [PubMed]
- Agha RA, Al Omran Y, Wellstead G, et al. Systematic review of therapeutic nipple-sparing versus skin-sparing mastectomy. BJS Open 2019;3:135-45. [Crossref] [PubMed]
- De La Cruz L, Moody AM, Tappy EE, et al. Overall Survival, Disease-Free Survival, Local Recurrence, and Nipple-Areolar Recurrence in the Setting of Nipple-Sparing Mastectomy: A Meta-Analysis and Systematic Review. Ann Surg Oncol 2015;22:3241-9. [Crossref] [PubMed]
- Harness JK, Vetter TS, Salibian AH. Areola and nipple-areola-sparing mastectomy for breast cancer treatment and risk reduction: report of an initial experience in a community hospital setting. Ann Surg Oncol 2011;18:917-22. [Crossref] [PubMed]
- Coopey SB, Smith BL. The Nipple is Just Another Margin. Ann Surg Oncol 2015;22:3764-6. [Crossref] [PubMed]
- Bailey CR, Ogbuagu O, Baltodano PA, et al. Quality-of-Life Outcomes Improve with Nipple-Sparing Mastectomy and Breast Reconstruction. Plast Reconstr Surg 2017;140:219-26. [Crossref] [PubMed]
- Metcalfe KA, Cil TD, Semple JL, et al. Long-Term Psychosocial Functioning in Women with Bilateral Prophylactic Mastectomy: Does Preservation of the Nipple-Areolar Complex Make a Difference? Ann Surg Oncol 2015;22:3324-30. [Crossref] [PubMed]
- Cloyd JM, Hernandez-Boussard T, Wapnir IL. Outcomes of partial mastectomy in male breast cancer patients: analysis of SEER, 1983-2009. Ann Surg Oncol 2013;20:1545-50. [Crossref] [PubMed]
- Cardoso F, Bartlett JMS, Slaets L, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol 2018;29:405-17. [Crossref] [PubMed]
- Elmi M, Sequeira S, Azin A, et al. Evolving surgical treatment decisions for male breast cancer: an analysis of the National Surgical Quality Improvement Program (NSQIP) database. Breast Cancer Res Treat 2018;171:427-34. [Crossref] [PubMed]
- Bateni SB, Davidson AJ, Arora M, et al. Is Breast-Conserving Therapy Appropriate for Male Breast Cancer Patients? A National Cancer Database Analysis. Ann Surg Oncol 2019;26:2144-53. [Crossref] [PubMed]
- Lin AP, Huang TW, Tam KW. Treatment of male breast cancer: meta-analysis of real-world evidence. Br J Surg 2021;108:1034-42. [Crossref] [PubMed]
- Network NCC. Invasive Breast Cancer (Version 2.2023).
- Luini A, Gatti G, Brenelli F, et al. Male breast cancer in a young patient treated with nipple-sparing mastectomy: case report and review of the literature. Tumori 2007;93:118-20. [Crossref] [PubMed]
- Tari DU, Morelli L, Guida A, et al. Male Breast Cancer Review. A Rare Case of Pure DCIS: Imaging Protocol, Radiomics and Management. Diagnostics (Basel) 2021;11:2199. [Crossref] [PubMed]
- Noor L, McGovern P, Bhaskar P, et al. Bilateral DCIS following gynecomastia surgery. Role of nipple sparing mastectomy. A case report and review of literature. Int J Surg Case Rep 2011;2:106-8. [Crossref] [PubMed]
- Stone KS, Wapnir IL. Male Breast Cancer. In: Grobmyer SR. editors. Complex General Surgical Oncology. Hamilton: Decker; 2017.
- Quincey K, Williamson I, Winstanley S. 'Marginalised malignancies': A qualitative synthesis of men's accounts of living with breast cancer. Soc Sci Med 2016;149:17-25. [Crossref] [PubMed]
- Chichura A, Attai DJ, Kuchta K, et al. Male Breast Cancer Patient and Surgeon Experience: The Male WhySurg Study. Ann Surg Oncol 2022;29:6115-31. [Crossref] [PubMed]
- Smith BL, Coopey SB. Nipple-Sparing Mastectomy. Adv Surg 2018;52:113-26. [Crossref] [PubMed]
- Karin MR, Pal S, Ikeda D, et al. Nipple Sparing Mastectomy Technique to Reduce Ischemic Complications: Preserving Important Blood Flow Based on Breast MRI. World J Surg 2023;47:192-200. [Crossref] [PubMed]
- Yadav S, Karam D, Bin Riaz I, et al. Male breast cancer in the United States: Treatment patterns and prognostic factors in the 21st century. Cancer 2020;126:26-36. [Crossref] [PubMed]
- Zaenger D, Rabatic BM, Dasher B, et al. Is Breast Conserving Therapy a Safe Modality for Early-Stage Male Breast Cancer? Clin Breast Cancer 2016;16:101-4. [Crossref] [PubMed]
- Smith BL, Tang R, Rai U, et al. Oncologic Safety of Nipple-Sparing Mastectomy in Women with Breast Cancer. J Am Coll Surg 2017;225:361-5. [Crossref] [PubMed]
- Orzalesi L, Casella D, Santi C, et al. Nipple sparing mastectomy: Surgical and oncological outcomes from a national multicentric registry with 913 patients (1006 cases) over a six year period. Breast 2016;25:75-81. [Crossref] [PubMed]
- Krajewski AC, Boughey JC, Degnim AC, et al. Expanded Indications and Improved Outcomes for Nipple-Sparing Mastectomy Over Time. Ann Surg Oncol 2015;22:3317-23. [Crossref] [PubMed]
- Weber WP, Haug M, Kurzeder C, et al. Oncoplastic Breast Consortium consensus conference on nipple-sparing mastectomy. Breast Cancer Res Treat 2018;172:523-37. [Crossref] [PubMed]
- Brachtel EF, Rusby JE, Michaelson JS, et al. Occult nipple involvement in breast cancer: clinicopathologic findings in 316 consecutive mastectomy specimens. J Clin Oncol 2009;27:4948-54. [Crossref] [PubMed]
- Zhang H, Li Y, Moran MS, et al. Predictive factors of nipple involvement in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 2015;151:239-49. [Crossref] [PubMed]
- Amara D, Peled AW, Wang F, et al. Tumor Involvement of the Nipple in Total Skin-Sparing Mastectomy: Strategies for Management. Ann Surg Oncol 2015;22:3803-8. [Crossref] [PubMed]
- Tang R, Coopey SB, Merrill AL, et al. Positive Nipple Margins in Nipple-Sparing Mastectomies: Rates, Management, and Oncologic Safety. J Am Coll Surg 2016;222:1149-55. [Crossref] [PubMed]
- Brain K, Williams B, Iredale R, et al. Psychological distress in men with breast cancer. J Clin Oncol 2006;24:95-101. [Crossref] [PubMed]
- Grenader T, Goldberg A, Shavit L. Second cancers in patients with male breast cancer: a literature review. J Cancer Surviv 2008;2:73-8. [Crossref] [PubMed]
- Auvinen A, Curtis RE, Ron E. Risk of subsequent cancer following breast cancer in men. J Natl Cancer Inst 2002;94:1330-2. [Crossref] [PubMed]
- Tevlin R, Griffin M, Karin M, et al. Impact of Incision Placement on Ischemic Complications in Microsurgical Breast Reconstruction. Plast Reconstr Surg 2022;149:316-22. [Crossref] [PubMed]
Cite this article as: Anderson TN, Bao J, Ayala C, Wapnir I, Karin MR. Contemporary mastectomy options for male breast cancer: nipple-sparing and areolar-sparing mastectomy—a case series. Ann Breast Surg 2024;8:27.

