
Invasive carcinoma in recurrent breast fibroadenoma: a case report and literature review
Highlight box
Key findings
• Triple negative invasive carcinomas within or adjacent to the fibroadenomas are more likely to have nodal metastasis.
• Location of invasive carcinoma in relation to fibroadenoma, whether adjacent or within, does not correlate with the risk of nodal metastases.
• The size of the carcinoma in relation to the fibroadenoma does not correlate with the risk of nodal metastases.
• In most cases, the eventual treatment of carcinomas arising from fibroadenomas was no different from treatment of invasive breast carcinoma.
What is known and what is new?
• Fibroadenomas belong to a common benign entity known as fibroepithelial lesions. Invasive breast carcinoma arising from fibroadenomas are rare.
• In the last decade, there are 21 cases reported of invasive carcinomas arising from fibroadenomas. We report the first case of recurrent fibroadenoma with invasive lobular carcinoma.
What is the implication, and what should change now?
• Patients need to be educated and encouraged to perform breast self-examination.
• Clinicians need to stay vigilant as recurrent benign lumps may have the potential to harbour malignancy.
Introduction
Fibroepithelial lesions (FEL), in general, include fibroadenomas (FA) and less occurring phyllodes tumours (1). The FA is the commonest benign tumour of the breast, occurring most frequently in women of reproductive age (2). Grossly, the FA can be variable from rounded to lobulated, with or without encapsulated borders. Microscopically, it is a biphasic tumour consisting of stromal and epithelial components. The FA stroma is usually of low cellularity, with variable appearances of myxoid, fibroblastic or hyalinised types. Calcifications, pseudoangiomatous stromal hyperplasia (PASH) and in rare instances, carcinoma, could also be present (3). In accordance with the CARE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-24-29/rc), we present a rare case involving a 58-year-old woman who had a recurrence of a FA almost a decade after initial diagnosis with final histology reporting an unexpected presence of invasive lobular carcinoma (ILC) within the FA.
Case presentation
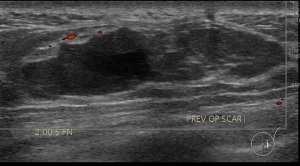
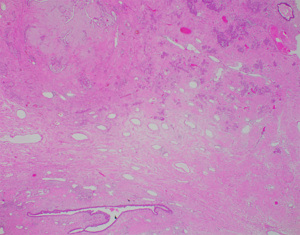
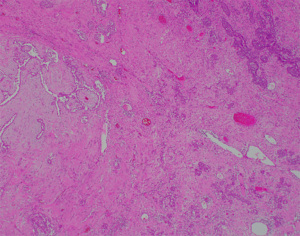
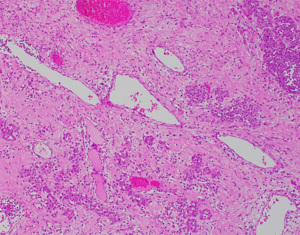
A 58-year-old lady who previously underwent an excision biopsy of a 5.5 cm FEL with PASH 10 years ago presents to clinic with a new left breast lump near the previous excision biopsy scar. Her last breast imaging was eight years prior and both ultrasound (US) and mammogram (MMG) of bilateral breasts at that time showed stable bilateral breast nodules with areas of architectural distortion of the left breast due to previous excision biopsy. Clinically, the new left breast lump was palpable at 1 o’clock and measured approximately 3 cm. There were no abnormalities with the overlying skin or nipple areolar complex. The right breast was normal on examination. There was no axillary lymphadenopathy bilaterally. US and MMG of both breasts showed that there was a large lobulated solid cystic mass in the left breast measuring 2.9 by 2.8 by 1.1 cm at 2 o’clock, 5 cm from nipple, adjacent to the previous excision biopsy scar (Figure 1). No axillary lymphadenopathy was noted. The patient proceeded with an US guided vacuum-assisted biopsy of the suspicious left breast solid-cystic mass. Histology later revealed grade 1 ILC. She decided to undergo a left simple mastectomy, and sentinel lymph node biopsy. Final histology confirmed the diagnosis of an 8 mm grade 1 ILC that was within a FA (Figures 2-5). There was no lymphovascular invasion and no involved lymph nodes. In view of T1bN0M0, stage 1A estrogen receptor (ER) positive, progesterone receptor (PR) positive, human epidermal growth factor receptor 2 (HER2) negative left breast cancer, she was recommended for adjuvant endocrine therapy at tumour board.
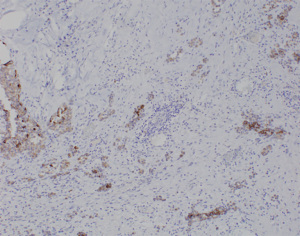
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In this study, we report the first case of a recurrent FA with ILC, despite previous excision biopsy of FEL/PASH in the same location. Whilst FAs are generally benign, predicting malignant transformation remains challenging. A literature review (summarized in Tables 1,2) from 2013–2023 revealed 21 other cases of breast cancer arising from FA. Similar to reported cases of FA with an invasive carcinoma component, patients tend to be older with a mean age of 45 years, the youngest patient being 26 years and the oldest patient being 64 years. In 11 cases, pre-operative biopsies showed carcinoma, while in 5 cases, pre-operative biopsies were benign. The other 6 cases did not report pre-operative biopsy results. Those with a benign pre-operative biopsy tend to have suspicious imaging findings on US or MMG. They include features of interval rapid growth in tumour size, irregular tumours with indistinct margins and abnormal calcifications that prompted surgical intervention. Most reported cases were early-stage tumours (63.6%), with HER2 negative tumours being most common (61.9%). The optimal management of carcinoma arising from FAs remains ambiguous, with current breast cancer guidelines offering limited guidance. It is unknown whether such patients should be treated similarly to those with invasive breast cancer. In terms of follow up, the minimum follow-up duration across all studies was 6 months and the maximum duration reported to be 30 months. There were no cases of recurrence and mortality reported from 2013 to 2023. Through literature review, we wish to discuss the (I) diagnostic challenges; (II) management approaches; and (III) phenomenon of carcinomas arising from FAs.
Table 1
| Author, year | Age (year) | Pre-op biopsy proven carcinoma | Histology | Size of FA/size of carcinoma (mm) | Carcinoma location with respect to fibroadenoma | Receptor status | Nodal metastasis | TNM stage | Neoadjuvant treatment | Surgery | Adjuvant treatment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | CH | ET | IT | |||||||||||
| Present case, 2023 | 58 | Y | ILC | 29/8 | Within | ER+ PR+ HER2− | N | cT2N0M0, pT1bN0M0 | N | Mastectomy and SLN | N | N | Y | N |
| Wang et al., 2022 (4) | 26 | N | LCIS with microinvasive lobular carcinoma | 32/5 | Within | ER− PR− HER2− | N | Un | N | Excision biopsy | Y | N | Y | N |
| Fujimoto et al., 2021 (5) | 31 | Y | IDC | 19/4 | Un | ER+ PR+ HER2+ | N | cT2N0M0, pT1aN0M0 | Offered CH but declined | BCS and SLN | Y | Y | N | Y |
| Shiino et al., 2020 (6) | 53 | Y | IDC | 36/Un | Within | ER− PR− HER2− | Y | cT2N1M0, pCR | CH | Mastectomy and AC | Y | N | N | N |
| Saadallah et al., 2019 (7) | 52 | N | IDC | Un/30 | Adjacent | ER− PR− HER2− | Y | cT2N0M0, pT2N1M0 | N | BCS and AC | Y | Y | N | N |
| Kothiya et al., 2018 (8) | 32 | Y | BLL | 55/Un | Within | Un | N | Un | CH for BLL | N | N | N | N | N |
| Ma et al., 2016 (9) | 51 | Y | IDC | 81/Un | Within | ER− PR− HER2− | Y | Un | N | Modified radical mastectomy | N | N | N | N |
| Aydın et al., 2015 (10) | 31 | Y | IDC | Un | Within | Un | N | Un | N | BCS and SLN | Y | Y | Y | Y |
| Park et al., 2015 (11) | 36 | N | DCIS | Un | Within | Un | N | Un | N | BCS and SLN | Y | N | N | N |
| Park et al., 2015 (12) | 39 | Y | IDC | 15/14 | Adjacent | ER+ PR+ HER2− | N | Un | N | BCS | Y | N | N | N |
| Myong et al., 2016 (13) | 61 | Y | MF | 50/Un | Within | Un | N | Un | N | BCS | N | N | N | N |
| Zheng et al., 2015 (14) | 48 | Y | IDC | Un/20 | Within | ER+ PR+ HER2+ | N | Un | N | Mastectomy and AC | N | Y | Y | N |
| Wu et al., 2014 (15) | 39 | N | IDC | 27/Un | Un | ER+ PR+ HER2− | N | T1aN0M0 | N | BCS and SLN | Refused | N | Refused | N |
| 31 | N | IDC | 35/Un | Un | ER+ PR+ HER2− | Y | T1aN1M0 | N | Mastectomy and AC | N | Y | N | N | |
| 30 | N | DCIS | 15/Un | Un | ER+ PR+ HER2− | N | TisN0M0 | N | BCS | N | N | N | N | |
| 63 | N | DCIS | 12/Un | Un | ER+ PR+ HER2− | N | TisN0M0 | N | BCS and SLN | Y | N | Y | N | |
| 48 | N | DCIS | 9/Un | Un | Un | N | TisN0M0 | N | Excision biopsy and SLN | N | N | N | N | |
| 40 | N | IDC | 6/Un | Un | ER+ PR− | N | T1bN0M0 | N | Mastectomy and SLN | N | N | N | N | |
| Iwamoto et al., 2014 (16) | 64 | Y | IDC | 20/7 | Adjacent | ER+ PR− HER2− | Y | Un | N | BCS and AC | N | N | N | N |
| Mele et al., 2014 (17) | 63 | Y | Invasive apocrine | 50/15 | Within | ER− HER2+ | Y | Un | N | Modified radical mastectomy | N | Y | N | N |
| Nonsefi et al., 2013 (18) | 58 | N | IDC | Un | Un | ER− PR− HER2− | N | Un | N | Excision biopsy (with 1 cm margins) | N | N | N | N |
| Hayes et al., 2013 (19) | 51 | N | LIN with microinvasive lobular carcinoma | 35/1 | Adjacent | ER+ HER2− | N | Un | N | Excision biopsy, re-excision for margins and SLN | N | N | N | N |
AC, axillary clearance; BLL, B acute lymphoblastic leukemia; CH, chemotherapy; DCIS, ductal carcinoma in situ; ER, estrogen receptor; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; H&E, hematoxylin and eosin; IDC, invasive ductal carcinoma; IT, immunotherapy; LIN, lobular intraepithelial neoplasia; MF, myofibrosarcoma; N, no; PR, progesterone receptor; RT, radiotherapy; SLN, sentinel lymph node biopsy; TNM, tumor, node, metastasis; Un, unknown; Y, yes; BCS, breast conserving surgery; FA, fibroadenoma; ILC, invasive lobular carcinoma; LCIS, lobular carcinoma in situ.
Table 2
| Tumour characteristics | Value |
|---|---|
| Pre-op biopsy proven carcinoma | |
| Yes | 11 (50.0) |
| No | 5 (22.7) |
| Unknown | 6 (27.3) |
| Histology | |
| Invasive ductal carcinoma | 12 (54.5) |
| Invasive lobular carcinoma | 3 (13.6) |
| Ductal carcinoma in situ | 4 (18.2) |
| Others | 3 (13.6) |
| Receptor status | |
| ER+ PR+ HER2– | 6 (27.3) |
| ER+ PR+ HER2+ | 2 (9.1) |
| ER− PR− HER2− | 5 (22.7) |
| ER− PR− HER2+ | 0 |
| Unknown | 9 (40.9) |
| Pathologic tumour stage | |
| Pathologic complete response | 1 (4.5) |
| 0 | 4 (18.2) |
| 1 | 8 (36.4) |
| 2 | 2 (9.1) |
| 3 | 0 |
| 4 | 1 (4.5) |
| Unable to be determined | 6 (27.3) |
| Nodal metastasis | |
| Yes | 6 (27.3) |
| No | 13 (59.1) |
| Unknown | 4 (18.2) |
| Breast surgery performed and adjuvant therapy | |
| Breast conserving surgery | 10 (45.5) |
| Mastectomy | 7 (31.8) |
| Excision biopsy | 4 (18.2) |
| None | 1 (4.5) |
| Axilla management | |
| Sentinel lymph node biopsy | 9 (40.9) |
| Axillary dissection | 7 (31.8) |
| None | 6 (27.3) |
| Adjuvant therapy | |
| Radiotherapy | 8 (40.0) |
| Chemotherapy | 5 (25.0) |
| Endocrine therapy | 5 (25.0) |
| Immunotherapy | 2 (10.0) |
| Size of fibroadenoma, mm | 24 [6–81] |
| Size of carcinoma, mm | 5 [4–30] |
Data are presented as mean [range] or n (%). ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Diagnostic challenges
In the literature review, out of 21 cases, 11 cases had malignant pre-operative biopsies, 5 cases had benign pre-operative biopsies while the other 6 cases did not report pre-operative biopsy results. Core needle biopsies can sometimes miss small malignancies, so surgical excision is advised when there is significant interval tumour growth or suspicious features on imaging. Imaging techniques, such as mammography and US may also fail to detect malignant changes within FAs particularly if the carcinoma is hidden by the benign tumour (20). Hence, thorough discussion with the patient about surgical excision for both diagnostics and potentially therapeutic purposes is warranted.
Management approaches
Management of carcinomas arising from FAs mirrors that of invasive breast cancer. For example, neoadjuvant therapy is used for triple-negative breast cancer (TNBC) and HER2 positive cases. Surgical options reported include excision biopsy, breast-conserving surgery (BCS), mastectomy and axilla surgery. There were 4 cases where excision biopsy was performed. Nahid et al. (18) reported a 1cm safe margin, while Wu et al. (15), Hayes et al. (19), Wang et al. (4) did not comment on oncological margins. Majority underwent BCS likely because clear surgical margins were feasible and patients were at an early stage. However, not all reported cases of BCS have undergone adjuvant radiotherapy (RT). For instance, Iwamoto et al. (16) and Myong et al. (13), both did not report or suggest adjuvant RT after BCS for their patients with invasive carcinoma with FA.
In management of invasive breast cancers, adjuvant therapy is determined by final pathological staging. Our case had a preoperative clinical staging of cT2N0 but final pathological staging is pT1bN0. This discrepancy between the gross lesion size and actual cancer size on histopathology (a gross lesion of 3 cm versus an eventual cancer size of 8 mm) should be discussed at a multidisciplinary team meeting to discuss whether the vacuum-assisted biopsy (VAB) had removed all the carcinoma component. If there is residual carcinoma, we need to enquire further if the remaining carcinoma is found within the VAB cavity or remains in its vicinity. This is to garner the closest estimation of the actual size of the invasive carcinoma component either as a summation or as a separate foci of cancer. It is also important to consider that the actual solid component rather than the large cystic component of the FA could also influence the final size of the invasive carcinoma and final staging. As adjuvant therapy is determined by the final pathological staging, it is crucial to accurately assess this to determine the appropriate course of treatment.
Although previous studies have shown that conditions such as FEL and PASH do not significantly increase the risk of future malignancies (21), any development of indeterminate or suspicious lesions in patients with these benign conditions should be thoroughly sampled with the least invasive approach such as core needle biopsy (CNB) or VAB in the case of solid cystic or cystic lesions. While some sources may recommend upfront surgical excision biopsy, current literature supports the effectiveness of VAB and vacuum-assisted excision (VAE) in sampling breast imaging and reporting and data system (BIRADS) 3 lesions as potentially providing a more accurate diagnosis while minimizing patient risk (22,23).
The phenomenon of invasive carcinoma adjacent or within FAs
The occurrence of carcinoma within complex FAs is rare. A concomitant carcinoma could be infiltrating an adjacent FA or an in situ malignant conversion of an epithelial component that occurred within a complex FA giving rise to an invasive carcinoma. In the literature review, 4 cases reported carcinoma adjacent to the FA while 8 cases found carcinoma within FA. Three out of the 8 cases where carcinoma was found within FA had nodal metastases whilst 2 out of 4 cases where carcinoma was found adjacent to FA had nodal metastases. The location of the carcinoma in relation to the FA, either adjacent or within the FA does not seem to correlate with the risk of nodal metastasis. From the literature review, it is also inconclusive regarding whether the location of cancer relative to the FA affects recurrence risk. Further research could explore whether the location of the invasive carcinoma in relation to the FA affects recurrence risk. The exact pathogenesis as to why and how carcinoma might arise from or adjacent to FAs remains unclear and warrants further exploration.
Conclusions
This report highlights the need for vigilance in monitoring patients with a history of FAs. Regular breast self-examinations are essential, as demonstrated by this case where our patient discovered a new lump near her previous excision biopsy scar a decade later. Despite the benign nature of FAs, patients with a history of such lumps can still develop malignant lesions. Inadequate sampling might provide false reassurance to both patients and clinicians, leading to an inaccurate diagnosis. Clinicians must adopt a proactive approach to monitoring patients, especially those with a family history of breast cancer or a history of FAs. The management of carcinomas arising from benign conditions should follow established protocols for invasive breast cancer, ensuring appropriate treatment and follow-up.
Acknowledgments
This case has been presented as a poster presentation in the global breast cancer conference (GBCC) in Seoul, Korea 2024.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-24-29/rc
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-24-29/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-24-29/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lerwill MF, Lee AHS, Tan PH. Fibroepithelial tumours of the breast-a review. Virchows Arch 2022;480:45-63. [Crossref] [PubMed]
- Loke BN, Md Nasir ND, Thike AA, et al. Genetics and genomics of breast fibroadenomas. J Clin Pathol 2018;71:381-7. [Crossref] [PubMed]
- Ajmal M, Khan M, Van Fossen K. Breast Fibroadenoma. Treasure Island (FL): StatPearls Publishing; 2024.
- Wang M, Leong MY, Tan QT. A case of fibroadenoma with lobular carcinoma in situ and microinvasion in a young woman. J Surg Case Rep 2022;2022:rjac602. [Crossref] [PubMed]
- Fujimoto A, Matsuura K, Kawasaki T, et al. Early HER2-positive breast cancer arising from a fibroadenoma: a case report. Oxf Med Case Reports 2021;2021:omab083. [Crossref] [PubMed]
- Shiino S, Yoshida M, Tokura M, et al. Locally advanced triple negative breast cancer arising from fibroadenoma with complete response to neoadjuvant chemotherapy: A case report. Int J Surg Case Rep 2020;68:234-8. [Crossref] [PubMed]
- Saadallah F, Bouraoui I, Naija L, et al. Coexistence of invasive ductal breast carcinoma and fibroadenoma. Pan Afr Med J 2019;33:139. [Crossref] [PubMed]
- Kothiya M, Shet T, Joshi S, et al. B acute lymphoblastic leukemia/lymphoma involving pre-existing fibroadenoma of the breast. Breast J 2018;24:82-3. [Crossref] [PubMed]
- Ma XL, Kang L, Li BJ, et al. Invasive Ductal Carcinoma Displayed "Basal-Like" Feature Arising within a Breast Fibroadenoma. Breast J 2016;22:695-6. [Crossref] [PubMed]
- Aydın OU, Soylu L, Ercan Aİ, et al. Invasive Ductal Carcinoma Developing From Fibroadenoma. J Breast Health 2015;11:195-8. [Crossref] [PubMed]
- Park EK, Cho KR, Seo BK, et al. Radiologic Findings of Ductal Carcinoma in Situ Arising Within a Juvenile Fibroadenoma: Mammographic, Sonographic and Dynamic Contrast-Enhanced Breast MRI Features. Iran J Radiol 2015;12:e17916. [PubMed]
- Park CJ, Kim EK, Woo HY, et al. Breast Cancer Arising Adjacent to an Involuting Fibroadenoma: Serial Changes in Radiologic Features. J Breast Cancer 2015;18:291-5. [Crossref] [PubMed]
- Myong NH, Min JW. Low-grade myofibroblastic sarcoma arising in fibroadenoma of the breast-A case report. Diagn Pathol 2016;11:33. [Crossref] [PubMed]
- Zheng H, Chen J, Wu X, et al. Bilateral breast cancer with a unilateral carcinoma within a fibroadenoma: A case report. Oncol Lett 2015;10:1513-6. [Crossref] [PubMed]
- Wu YT, Chen ST, Chen CJ, et al. Breast cancer arising within fibroadenoma: collective analysis of case reports in the literature and hints on treatment policy. World J Surg Oncol 2014;12:335. [Crossref] [PubMed]
- Iwamoto M, Takei H, Iida S, et al. Contralateral breast cancer adjacent to a fibroadenoma: report of a case. J Nippon Med Sch 2014;81:168-72. [Crossref] [PubMed]
- Mele M, Vahl P, Funder JA, et al. Apocrine carcinoma arising in a complex fibroadenoma: a case report. Breast Dis 2014;34:183-7. [Crossref] [PubMed]
- Monsefi N, Nikpour H, Safavi M, et al. Mucinous subtype of invasive ductal carcinoma arising within a fibroadenoma. Arch Iran Med 2013;16:366-8. [PubMed]
- Hayes BD, Quinn CM. Microinvasive lobular carcinoma arising in a fibroadenoma. Int J Surg Pathol 2013;21:419-21. [Crossref] [PubMed]
- Bilous M. Breast core needle biopsy: issues and controversies. Mod Pathol 2010;23:S36-45. [Crossref] [PubMed]
- Nassar A, Visscher DW, Degnim AC, et al. Complex fibroadenoma and breast cancer risk: a Mayo Clinic Benign Breast Disease Cohort Study. Breast Cancer Res Treat 2015;153:397-405. [Crossref] [PubMed]
- van de Voort EM, Struik GM, Birnie E, et al. Implementation of vacuum-assisted excision as a management option for benign and high-risk breast lesions. Br J Radiol 2023;96:20220776. [Crossref] [PubMed]
- Elfgen C, Leo C, Kubik-Huch RA, et al. Third International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Virchows Arch 2023;483:5-20. [Crossref] [PubMed]
Cite this article as: Ho MY, Ng V, Hing J. Invasive carcinoma in recurrent breast fibroadenoma: a case report and literature review. Ann Breast Surg 2025;9:16.

