
Exploring the association between post-operative complications, disease-recurrence & mortality in primary operable breast cancer—systematic review & meta-analysis
Highlight box
Key findings
• Post-operative complications (POCs) after breast cancer surgery are associated with increased disease-recurrence and all-cause mortality.
• Increased patient age and mastectomy over breast conservation surgery, increase the risk of POC with associated inferior survival.
What is known and what is new?
• Previous work suggested a likely association between POC and outcomes but noted significant variability in the evidence.
• We present stronger evidence of an association between POCs and increased mortality in primary breast cancer.
What is the implication, and what should change now?
• This meta-analysis further emphasises the need for de-escalation in breast cancer surgery. Where possible conservation surgery should always be the preferred surgical option to reduce the incidence of POC and the impacts on oncological outcomes and survival; especially in older women.
• Further data exploring the incidence of POC and oncological outcomes in oncoplastic breast surgery and differing reconstructive techniques is crucial to inform shared decision-making with patients who do require mastectomy.
• Further work exploring cellular changes, and the underlying mechanism is required to begin development of personalized patient treatments to mitigate risk.
Introduction
Background
Breast cancer affects 2.3 million women globally per annum (1). Surgery with curative intent remains the standard of care, supported by adjuvant treatments. Despite improvements in breast cancer screening and treatment over the past two decades, 670,000 women died from breast cancer-related causes in 2022 (1). Cancer recurrence remains a dominant contributor to breast cancer-related deaths (2). Breast cancer recurrence can occur as local disease at the same site, regional disease within the lymph node structures or chest wall periphery, distal disease metastases to a different anatomical site or a combination of all three. When breast cancer recurs, 5-year survival rate can drop to less than 30% (3). The risk of 10-year recurrence varies depending on cancer subtype and adjuvant therapy, and ranges from 4–34% (4). The recent James Lind Alliance Priority Setting demonstrates the importance to both patients and clinicians of exploring risk factors for breast cancer recurrence, identifying higher-risk patients, and helping them to make more informed decisions regarding breast cancer treatment (5).
Post-operative complications (POCs), defined as ‘any deviation from the normal post-operative course’, affect up to 36.1% of breast cancer patients (6,7). POCs are often described as minor, managed in an outpatient setting with wound care, dressings or antibiotics; or major requiring intervention and/or readmission to hospital (Clavien-Dindo grade III and above) (6). Risk factors for developing POCs are well described in the literature and include patient-specific (age; obesity; comorbidities; smoking history), surgical (breast-conservation vs. mastectomy; concurrent reconstruction ± prosthesis) or environmental factors (skin preparation; peri-operative antibiotics; wound care/dressings) (8). POCs cause patients’ pain and discomfort, can prolong initial hospital admission and/or necessitate readmission with associated health-care costs. In addition, POCs can cause a delay in adjuvant treatments, increase risk of disease-recurrence and, most importantly, have been associated with inferior overall and cancer-specific survival (7,9,10).
Evidence of the effect of surgical complications has been described for other cancers. The COLOR I and II trials identified that patients who experience an anastomotic leak following surgery for colorectal cancer are at higher risk of local disease-recurrence [hazard ratio (HR) 2.96, 95% confidence interval (CI): 1.38–6.34, P=0.005] and mortality (HR 1.67, 95% CI: 1.16–2.41, P=0.006) (11). Similar outcomes have been described in gastro-oesophageal cancer, lung cancer, and head and neck cancers (12-14). Systemic POCs, for example post-operative pneumonia, have also been demonstrated to correlate with inferior survival outcomes (15).
The underlying mechanism is complex with multiple synergistic pathways (16). Excision of a solid tumour can release malignant cells into the bloodstream. At the same time, surgical trauma stimulates inflammatory cytokines and chemokines, such as tumour necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), to be released into the circulation. These mediators can interact with residual tumour cells, both locally and in dormant distant metastatic deposits to propagate tumour proliferation (10). Pro-inflammatory cytokines have also been shown to stimulate the adhesion of circulating cancer cells capturing tumour cells in foreign locations, hence promoting metastases (16,17). During wound healing, additional inflammatory mediators are released alongside activated macrophages, fibroblasts, smooth muscle cells and keratinocytes. These factors regulate the differentiation, growth, and migration of all cells in the tumour environment, and it has been shown that tissue factors associated with wound healing can stimulate and contribute to tumour growth (10). Prolonged wound healing over a long period of time can even result in cancer development de novo in certain conditions, and thus Dvorak described cancer as ‘the wound which never heals’ (18). In addition, effects of anaesthesia and post-surgical stress create a ‘window’ of immunosuppression which can reduce the body’s ability to eliminate residual malignant cells. Interferon-gamma (IFN-γ), a cytokine integral in controlling metastases, is suppressed for up to two months following colorectal cancer surgery, whilst the total number of active T-cells and natural killer (NK) cells are reduced after lung, gastric and renal cancer surgery for up to two weeks (16). In the context of a POC, wound healing and the immunosuppression window are prolonged which can lead to increased growth of occult micro-metastases (19).
Rationale and knowledge gap
The impact of POCs on disease-recurrence and survival is less well defined in breast cancer. We previously carried out a meta-analysis with inconclusive results (19). With the emergence of new evidence, we provide an update of the best level evidence and repeat meta-analysis for disease-recurrence and mortality following POC after surgery for primary invasive breast cancer.
Objective
The aim of this review was to identify, appraise and summarise the current literature for patients who suffer postoperative complications after breast cancer surgery, and to establish how this impacts disease-recurrence and mortality. We present this article in accordance with the PRISMA reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-24-35/rc).
Methods
A literature search was conducted on 1st February 2024 and is summarized in Table 1. The search strategy was developed based on the eligibility criteria in collaboration with a specialist librarian. Articles were screened against pre-defined inclusion and exclusion criteria, and the bibliographies of suitable studies were hand-searched for additional eligible studies. Original articles published in English after 1990 analysing oncological outcomes after the development of POCs in primary breast cancer were included. Articles focusing on risk factors for development of POCs, or those assessing in situ or metastatic disease were excluded, as were any secondary review papers. The screening process is summarized in Figure 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 1st February 2024 |
| Database searched | PubMed |
| Search terms used | “Breast Neoplams”[Mesh], “breast cancer”[tw], “breast neoplasm(s)”[tw], “breast carcinoma”[tw], Mastectomy”[Mesh], “Mammaplasty”[Mesh], “breast surger*”[tw], “breast conserv*”[tw], mastectom*[tw], mammoplast*[tw], surg*[tw], operable[tw], “Postoperative Complications”[Mesh:NoExp], “Surgical Wound Infection”[Mesh], “Surgical Wound Dehiscence”[Mesh], “Fever”[Mesh:NoExp], “Hematoma”[Mesh:NoExp], “Inflammation”[Mesh], “Necrosis”[Mesh:NoExp], “Inflammation Mediators”[Mesh], “postoperative complicat*”[tw], “postoperative infect*”[tw], “surgical site infect*”[tw] OR fever[tw], haematoma*[tw], necrosis[tw], inflammation[tw], “Survival“[Mesh], “Recurrence“[Mesh], “Neoplasm Recurrence, Local“[Mesh], survival[tw], recurren*[tw] |
| See example of search strategy in PubMed (Table S1) | |
| Timeframe | 2007–2023 |
| Inclusion criteria | Human studies; English language; Original articles; Published after 1990 |
| Selection process | Search strategy & selection criteria agreed between M.M./L.A./J.E./L.R.; selection conducted independently by M.M. |
| Any additional considerations | Bibliographies of selected papers hand-searched for additional suitable papers (n=4) |
tw, text word.
Eligible studies were evaluated using the Newcastle-Ottawa Scale (20). Data collected from each study included year of publication and geographical location, study design, inclusion criteria, sample size, definition of complications, median follow-up, and any reported outcomes for time to adjuvant treatment, disease-recurrence (locoregional/systemic/any site) and mortality. We reviewed the evidence in relation to risk factors for developing POCs (patient factors; surgical factors; type of complication), before reviewing the oncological outcomes (time to adjuvant treatment; disease-recurrence; mortality).
Statistical analysis
Meta-analysis for disease-recurrence and survival was performed using Review Manager Web version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark). Only studies which performed multivariate analysis were included. HRs for each outcome, from each eligible study were combined using a random effects model to account for heterogeneity of methodology and complication type. Heterogeneity was assessed using the I2 test and two-tailed P values <0.05 were considered statistically significant. Results were presented using a forest plot.
Results
Original search identified a total of 2,351 articles. After screening the journal title and abstract, a full paper analysis was carried out on 79 papers. A further 61 were excluded due to the focus on risk factors for the development of POCs: patient factors [14]; surgical risk factors [42]; tumour factors [3] and adjuvant treatment [2]. In addition, one paper published before 1990 was excluded, one paper was subsequently retracted and a further four secondary review papers were also excluded. The bibliographies of the remaining 12 papers were hand-searched for additional eligible studies, of which four were identified. Sixteen studies were included in the final review and are summarised in Table 2.
Table 2
| Study, country | Published year | Study type | Patient cohort | Definition of complication | Median follow-up (months) | Outcomes relating to POC | Newcastle Ottawa Scale | ||
|---|---|---|---|---|---|---|---|---|---|
| Time to adjuvant tx | Recurrence | Mortality | |||||||
| Indelicato et al. (21), USA | 2007 | Retrospective, single-centre | Stage 0–II IBC undergoing BCS + RTx (n=516) | Acute breast infection (features of erythema, tenderness, warmth) within 3 months post-surgery | 76.8 | Delayed in 45.2% | True local recurrence: P<0.001; ipsilateral recurrence: P=0.03 | – | 5 |
| Murthy et al. (9), UK | 2007 | National cancer registry retrospective, single-centre | Unilateral T1–4N0–3M0 IBC (n=1,065) | Wound complications requiring surgical debridement, dressing, packing or persistent discharge before completion of adjuvant treatment | 54 | No significant delay | Adjusted HR (95% CI) of systemic recurrence 2.52 (1.69–3.77), P<0.001 | – | 9 |
| Yan et al. (22), China | 2010 | Retrospective, single-centre | Unilateral node-negative IBC (n=883) | Post-op oral fever >38 ℃ within 7 days post-surgery | 43 | – | Adjusted RR (95% CI) of locoregional/systemic recurrence 1.89 (1.02–3.52), P=0.04 | – | 6 |
| de Glas et al. (23), Netherlands | 2013 | Population cohort, multi-centre (FOCUS study) | Stage I–IV in situ & IBC >65 years (n=3,179) | Wound-related & non-wound-related complications requiring treatment within 30 days post-surgery | 86.4 | – | – | Adjusted HR (95% CI) for all-cause mortality 1.21 (1.07–1.36), P=0.002 | 8 |
| Beecher et al. (24), UK | 2016 | Retrospective tertiary centre | IBC undergoing Mx + IBR (n=229) | Wound complications within 30 days post-surgery (infection/haematoma/flap dehiscence/skin necrosis) | 80 | – | Adjusted HR (95% CI) of locoregional/systemic recurrence 4.61 (2.47–8.61), P<0.001 | – | 8 |
| Pedersen et al. (25), Denmark | 2017 | Population cohort study | Stage I–III IBC undergoing BCS or Mx (n=30,711) | Post-operative bleeding, requiring surgery, within 14 days IBC surgery | 84 | – | Adjusted HR (95% CI) of locoregional/systemic recurrence 1.06 (0.89–1.26) | – | 9 |
| Mousa et al. (26), Israel | 2018 | Retrospective, single-centre | IBC undergoing Mx + IBR (n=186) | Wound complications within 90 days post-surgery | 37.4 | 60 vs. 47 days (no significant delay) | No significant association | – | 6 |
| Abdullah et al. (27), UK | 2019 | Retrospective, single-centre | IBC undergoing Mx + IBR (n=107) | Wound complication (infection/haematoma/skin necrosis/implant removal) | 47 | – | Locoregional/systemic: P=0.69 | All-cause mortality: P=0.49 | 4 |
| Valente et al. (28), USA | 2019 | Retrospective, single-centre | Stage I–III IBC undergoing Mx + IBR (n=458) | Wound complication, requiring intervention, within 60 days post-surgery | 90 | 52 vs. 41 days, P<0.001 | Adjusted HR (95% CI) of locoregional/systemic recurrence 1.51 (0.64–3.36), P=0.33 | – | 6 |
| Lee et al. (29), South Korea | 2020 | Retrospective, Tertiary centre | Stage 0–II BC undergoing Mx + IBR (n=438) | Breast wound complications requiring intervention, and donor site complications following autologous reconstruction | 82 | 35 vs. 32 days, P=0.17 | Adjusted HR (95% CI) for locoregional/systemic recurrence: 2.25 (1.17–4.33), P=0.02 | – | 7 |
| Hammond et al. (30), USA | 2020 | Retrospective, single-centre | Stage I–III primary IBC undergoing Mx ± IBC (n=378) | Surgical and non-surgical complications | 60 | 76 vs. 75 days, P=0.64 | Unadjusted HR (95% CI) for locoregional/systemic recurrence: Mx 1.45 (0.50–4.19), P=0.74; Mx + IBR 1.10 (0.33–3.69), P=0.99 | Unadjusted HR (95% CI) for all-cause mortality: Mx 1.66 (0.82–3.36), P=0.35; Mx + IBR 0.78 (0.27–2.27), P=0.13 | 6 |
| Siegel et al. (31), USA | 2021 | Retrospective, single-centre | Primary IBC undergoing Mx + IBR (n=201) | Wound complications (haematoma, implant-related, infection, flap necrosis, nipple necrosis and wound dehiscence) | 106.8 | Chemotherapy: P=0.71; RTx: P=0.14 | Unadjusted HR (95% CI) for locoregional/systemic recurrence 2.23 (0.88–5.63), P=0.05 | Unadjusted HR (95% CI) for all-cause mortality 1.24 (0.21–7.50), P=0.81 | 6 |
| Adwall et al. (32), Sweden | 2021 | Retrospective, multi-centre | IBC undergoing BCS or Mx (n=492) | Surgical site infection or any other post-operative infection within 90 days surgery | 100.8 | – | Adjusted HR (95% CI) for systemic recurrence 0.99 (0.41–2.34), P=0.97 | – | 8 |
| Londero et al. (33), Italy | 2022 | Retrospective single-centre | F, breast surgery (n=5,039) | Venous thromboembolism within 3 months breast surgery | 75 | – | – | Unadjusted HR (95% CI) for all-cause mortality: 5.3 (3.3–8.6), P=0.05 | 7 |
| de Boniface et al. (7), Sweden | 2022 | Population cohort study | T1–3N0–3M0 IBC undergoing BCS + RTx and Mx ± IBC ± RTx (n=57,152) | Major surgical complications requiring readmission or reoperation within 30 days post-surgery | 74.6 | – | – | Adjusted HR (95% CI) for all-cause mortality 1.32 (1.15–1.51); adjusted HR (95% CI) for cancer-specific mortality 1.31 (1.04–1.65) | 9 |
| de Boniface et al. (34), Sweden | 2023 | Population cohort study | T1–3N0–3 M0 IBC undergoing BCS + RTx or MX-RTx >50 years (n=34,139) | Major surgical complications requiring readmission or reoperation or medical complications withing 30 days post-surgery | 73.7 | – | – | Adjusted HR (95% CI) for all-cause mortality following major surgical complications: 50–69 years 1.92 (1.35–2.74), P<0.001; ≥80 years 1.43 (1.09–1.86), P=0.009. Adjusted HR (95% CI) for all-cause mortality following medical complications: ≥80 years 1.60 (1.25–2.06), P<0.001 | 9 |
IBC, invasive breast cancer; BCS, breast conservation surgery; Mx, mastectomy; IBR, immediate breast reconstruction; F, female; RTx, radiotherapy; tx, treatment; HR, hazard ratio; RR, relative risk; CI, confidence interval; POC, post-operative complication.
Patient factors
Extremes of age are a recognised risk factor for inferior prognosis in breast cancer (35). Two studies analysed oncological outcomes following POCs in an elderly cohort (23,34). The Dutch FOCUS study group analysed the impact of wound and non-wound related complications within 30 days post-surgery. Increased all-cause mortality was observed in patients who experienced a POC compared to those who did not have a complication (HR 1.21, 95% CI: 1.07–1.36, P=0.002) (23). de Boniface et al. analysed a Swedish patient cohort in three age categories: 50–69, 70–79, and ≥80 years. Increased mortality was observed in patients aged 50–69 years (HR 1.92, 95% CI: 1.35–2.74, P<0.001) and ≥80 years (HR 1.43, 95% CI: 1.09–1.86, P=0.009) who experienced a major surgical complication and in patients ≥80 years (HR 1.60, 95% CI: 1.25–2.06, P<0.001) who experienced a medical complication, compared to those who did not (34). Both are large, population-cohort studies with data extracted from a National Cancer Registry and follow-up of over 70 months. Survival outcomes were maintained after adjusted analysis for comorbidity, disease stage and grade, type of surgery and treatment adjuncts, as well as socio-economic background in the later Swedish study. Both studies reported an association between both localised surgical complications and systemic complications and increased mortality risk. Unfortunately, neither study accounted for recurrence rates or reported cancer-specific survival, and so we do not know if death was directly related to breast cancer. Other patient factors such as obesity and smoking status are associated with increased risk of POCs (9,28), but studies exploring associated oncological outcomes are lacking.
Surgical factors
The impact of surgical techniques and their effect on outcomes following POCs was the focus of the majority of papers included in this review, with immediate breast reconstruction the most common topic. Immediate reconstruction increases risk of developing POCs compared to mastectomy alone (30); however, the effect this has on oncological prognosis is less well understood. In addition, there is now significant variability in the reconstructive and oncoplastic techniques available, e.g., implant-based (pre-pectoral or sub-pectoral); autologous local flaps and/or free-flaps, each with different complication rates (36) adding a level of complexity when interpreting the data.
Five papers explored the correlation between mastectomy and immediate breast construction (Mx + IBR) and oncological outcomes (24,26,28,29,31), all of which were single-limb retrospective studies. Valente et al. reported a significant delay to adjuvant treatment in patients who experienced a POC following Mx + IBR (52 vs. 41 days, P<0.001) (28); three studies found no significant delay (26,29,31) and one study did not assess time to adjuvant treatment (24). Three of these studies demonstrate an association between POCs and disease-recurrence following Mx + IBR (24,29,31); one of which was borderline significant (HR 2.23, 95% CI: 0.88–5.63, P=0.05), with no impact on survival (HR 1.24, 95% CI: 0.21–7.50, P=0.81) (31). Whilst not the primary focus of their study, Hammond et al. included both mastectomy with or without reconstruction (Mx ± IBR) in their analysis (n=378). They demonstrated no significant delay to adjuvant treatment, and no impact on breast cancer recurrence or survival for those who experienced a POC compared with those who did not, regardless of concurrent reconstruction (30). Four of five studies included both implant-based and autologous IBR (24,28,29,31). When comparing complication rates associated with different techniques, two studies reported increased POC risk following implant-based reconstruction however this was lost on multivariant analysis in one (28) and not carried out in the other (31). One study reported complication rates following different techniques within these subgroups, but only reported comparisons between implant-based and non-implant-based reconstruction (P=0.54) (24).
de Boniface et al. analysed the association between major surgical POCs and mortality, and stratified patients by type of breast surgery. POCs rates were significantly higher following more extensive surgery (Mx + IBR 7.8% vs. mastectomy 4.3% vs. conservation surgery 2.3%, P<0.001), and patients who developed a POC after mastectomy were at increased risk of both all-cause (HR 1.32, 95% CI: 1.15–1.51) and cancer-specific (HR 1.31, 95% CI: 1.04–1.65) mortality. Increased risk associated with POC was not observed amongst patients undergoing conservation surgery. This is the largest population cohort study included in our review (n=57,152) and having adjusted for recognised prognostic factors as well as comorbidity and socio-economic status is strong evidence supporting increased overall and cancer-specific mortality following POCs, especially following mastectomy (7). Poor outcomes may be explained by the larger surgical insult associated with mastectomy compared to conservation surgery, consistent with evidence of poor outcomes following open compared to minimally invasive surgery in colorectal cancer (11). However, the larger surgical insult following Mx + IBR using autologous donor sites has not been associated with increased mortality. In a study of 57,152 subjects, de Boniface et al. included patients undergoing Mx + IBR; despite a higher rate of POCs, only one death was observed amongst this group (7).
Type of complication
Wound complications
Most studies in our review assess for wound complications, the definition of which varied between studies. Some studies defined their wound complication by severity, for example requiring intervention or readmission (7,25,28,29,34); others defined by infective vs. non-infective aetiology (21,32), and others used timeframe as a marker (9,26). Examples of recorded wound complications include surgical site infection (SSI), bleeding or haematoma, skin or nipple necrosis, and seroma. This heterogeneity accounts for the range of reported rates of wound complications (2.5–49%) and makes comparison between studies challenging.
Three studies assessed SSI (21,27,32) and its association with oncological outcomes and a further two studies carried out a subgroup analysis on ‘wound infection’ or similar (24,31). An early study of 516 patients found an association between acute breast infection and both true local (P<0.001) and ipsilateral breast cancer recurrence (P=0.03) (21), however, more recent results have found conflicting results (27,32). The SSI rate varied between studies (7.4–17.8%), which is reflective of retrospective data collection as well as the defined criteria. In their subgroup analysis, Beecher et al. identified a significant association between post-operative wound infection and breast cancer recurrence after adjusting for Nottingham Prognostic Index (NPI), lympho-vascular invasion, chemotherapy, and radiotherapy (P<0.001) (24). Meanwhile, Siegel et al. did not identify an association between SSI and disease-free survival (HR 1.1, 95% CI: 0.25–4.76, P=0.99) (31). We should also acknowledge that, although, no subgroup analysis was undertaken, wound infection was one of the two most common complications in the FOCUS study where higher risk of mortality was observed in patients who developed a POC within 30 days post-surgery (23).
Pedersen et al. analysed the impact of post-operative bleeding requiring repeat surgery within 14 days of their initial operation. In a large population study of 30,711 patients, post-operative bleeding rates were low (2.5%) and no association with disease-recurrence was observed (HR 1.06, 95% CI: 0.89–1.26) (25). No other studies have studied the impact of independent wound complications.
Non-breast wounds for example axillary or autologous donor sites are at equal risk of developing a wound complication. Lee et al. considered the impact of donor site wound complications infections after Mx + IBR. About 6.8% women (n=30) were affected but this did not affect disease-free survival compared to patients without complications on multivariant analysis (HR 0.91, 95% CI: 0.21–4.01) (29). No other studies compared axillary and breast wounds as separate complications. Axillary node clearance is associated with increased risk of POCs and inferior patient reported outcomes. This was identified in three large population studies included this review, and appropriately accounted for in multivariant survival outcome analysis (7,23,34).
Non-wound related complications
Two studies in our review looked at non-wound related complications. Yan et al. assessed for post-operative fever within 7 days post-surgery in unilateral, node-negative breast cancer (22). They found a significant correlation between post-operative fever and breast cancer recurrence at an average of 43-month follow-up after adjustment for age, co-morbidity, tumour size and receptor status, and type of surgery [relative risk (RR) 1.89, 95% CI: 1.02–3.52, P=0.04]. This was associated with inferior overall 5-year survival (P=0.003). They propose a theoretical systemic response which stimulates the pro-inflammatory process to exacerbate pre-existing sub-clinical lesions and confer resistance to systemic treatments. Whilst this is the only study to demonstrate a link between post-operative fever and increased risk of recurrence in breast cancer, the exact mechanism of how they captured post-operative fever rate is unclear. In addition, this study was carried out in an Asian population in China and thus we do not know if this is relevant to patients in other countries.
Londero et al. analysed venous thromboembolism (VTE) following breast surgery and demonstrated inferior overall survival amongst patients with stage I–III breast cancer who experience a VTE event after breast cancer surgery (HR 5.3, 95% CI: 3.3–8.6, P=0.05) (33). Survival outcomes were equivocal in patients with stage IV disease. In this study, all cancer patients were given 28 days VTE-prophylaxis. This is no longer common practice worldwide and may have reduced rates of detectable VTE in the cancer cohort.
Time to adjuvant treatment
Guidelines vary with regards to appropriate time to adjuvant treatment following primary breast cancer surgery (37). Timely access to adjuvant treatments can down-regulate the post-surgical stress response and inflammatory pathways and prevent early micro-metastases. The window of host ‘immunosuppression’ further enables the physicians to control the host response and is the target of many novel therapies (16).
Seven studies explored the effect of POCs and time to adjuvant treatment (9,21,26,27,29,30,32). Indelicato et al. found a delay to radiotherapy, defined as more than six weeks post-surgery in the absence of adjuvant chemotherapy, in 45.2% patients who experienced acute breast infection after breast cancer surgery. Amongst this group, there were significantly higher rates of true local (within the same tumour bed, P<0.001) and ipsilateral recurrence (tumour occurring elsewhere within the same breast, P=0.03) (21). This study included patients with stage 0–II disease who underwent breast conservation surgery; however, patients with in situ disease (stage 0) were removed from recurrence analysis to avoid bias. All patients who failed local control of acute breast infection after breast cancer surgery recurred in the same tissue bed, or tissue adjacent to the original tumour bed. The authors hypothesise that the acidic environment created during an acute breast infection increases extracellular activity of cathepsin D, a specific protease involved in the process of tumour cell migration in the breast; this effect is then synergised by a delay to adjuvant treatment. Valente et al. assessed delay to adjuvant treatment in patients with stage I–III disease undergoing Mx + IBR. They found significant delay to adjuvant treatments in patients who experience wound complications requiring intervention (52 vs. 41 days, P<0.001) yet this did not increase breast cancer recurrence rates (HR 1.51, 95% CI: 0.64–3.36, P=0.33) (28). Whilst these two studies of similar size suggest POCs can delay adjuvant treatment in both early and more advanced breast cancer, the sequential impact on disease-recurrence are conflicting. Five other retrospective, single-centre studies identified no significant delay to adjuvant treatments in patients experiencing a POC following breast cancer surgery (9,26,29-31). Three of which demonstrate increased risk of recurrence in patients who experienced a POC (9,29,31) albeit one was borderline significant (31), whilst two others demonstrated no association (26,30). Lee et al. carried out a subgroup analysis comparing patients who received adjuvant treatment (n=200) and those who did not (n=238). Amongst patients who did not receive adjuvant treatment, development of a POC did not significantly impact breast cancer recurrence (HR 2.26, 95% CI: 0.72–7.04, P=0.16). However, amongst the patients who did receive adjuvant treatment, development of a POC was associated with increased risk of recurrence after adjustment for other variables including time to adjuvant therapy (HR 2.29, 95% CI: 1.07–4.90, P=0.03). To further reduce any confounding effects, the authors carried out additional analysis excluding patients who started adjuvant treatment more than 8 weeks after surgery. Time to initiation of adjuvant treatment did not differ significantly between patients with and without complications (34 vs. 32 days, P=0.22) and association between development of a POC and recurrence was observed after adjustment for other variables (HR 2.5, 95% CI: 1.09–5.52, P=0.03) (29).
Disease-recurrence
Large, high grade, node-positive tumours and omission of adjuvant radiotherapy are associated with increased risk of breast cancer recurrence (16). In addition, triple-negative and human epidermal growth factor receptor 2 (HER2) positive tumours are associated with highest rates of recurrence at 10-years after adjustment for baseline patient and tumour characteristics, as well as treatments received (38).
Twelve studies explored the relationship between POCs and disease-recurrence (9,21,22,24-32). One paper looked at local recurrence only (21) and one looked at systemic recurrence only (32). Murthy et al. was the first study to explore this association and remains one of the largest published studies (9). In a National Cancer Registry of 1,065 patients, 9% patients experienced post-operative wound complications requiring treatment or further intervention. Primary tumours of all sizes irrespective of nodal involvement were included in this study, and rates of systemic recurrence were reported with median follow-up of 54 months. A near 3-fold difference in distal recurrence rates was observed in patients who experienced a POC compared to those who did not, and this significance was maintained after adjustment for patient age, smoking status, type of surgery, hormone receptor status and NPI status (HR 2.52, 95% CI: 1.69–3.77, P<0.001). The authors also reviewed local recurrence rates, but these were not significant. Systemic recurrence rates were not explained by recognised risk factors including type of surgery or smoking status, and the authors hypothesise that causative factors exist at a cellular level. These data differ from observations in colorectal cancer whereby localised complications translate into local disease-recurrence (11). However, adjuvant treatments which act to prevent disease-recurrence have changed dramatically since the study period (1994–2001) and thus, results should be interpreted accordingly. A more recent study by Adwall et al. identified an association between SSI and the crude rate of systemic recurrence (HR 1.97, 95% CI: 1.04–3.76, P=0.04), but this effect was not sustained following multivariant analysis (HR 0.99, 95% CI: 0.41–2.34, P=0.97) (32). This study used similar wound complication criteria to Murthy et al. defining an SSI as requiring treatment with antibiotics and/or surgical dressing, debridement or drainage (9). Other systemic infection, for example chest or urinary tract, was not a significant risk factor for systemic recurrence (HR 1.57, 95% CI: 0.73–3.30, P=0.25). One other study analysed the correlation between acute breast infection in early, stage 0–III breast cancer and local recurrence. In a cohort of 516 patients, both true local and ipsilateral breast cancer recurrence (P<0.001 and P=0.03 respectively) were more common following acute breast infection (21). This observation has not been demonstrated in more advanced breast cancer.
Four papers conclude that there is an association between POCs and breast cancer recurrence at any site (22,24,29,31), whilst five papers found no relationship (25-28,30). All studies are retrospective and of varying sample size, though one large population study is included amongst the five papers where no relationship was identified (n=30,711) (25). In addition, methodology for identifying breast cancer recurrence varies between these studies.
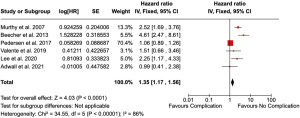
Six studies carried out multivariant analysis assessing risk of disease-recurrence after POCs and were eligible for meta-analysis (9,24,25,28,29,32). Newcastle Ottawa Score ranged from 6–9. Meta-analysis of 33,393 patients demonstrated a significant effect of complications in risk of recurrence (HR 1.35, 95% CI: 1.17–1.56, P<0.001), though a high degree of heterogeneity was noted (I2=86%) (Figure 2).
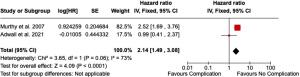
Two of these studies assessed risk of systemic recurrence only after POCs (9,32). Meta-analysis of 1,557 patients demonstrated a significant impact of complications on systemic recurrence (HR 2.14, 95% CI: 1.49–3.08, P<0.001) however the degree of heterogeneity remains high (I2=73%) (Figure 3).
Mortality
Seven of our studies assess the association between POCs and mortality following breast cancer surgery (7,23,27,30,31,33-34), only one of which reports cancer-specific mortality independently of all-cause mortality (7). de Boniface et al. published two separate papers from the same population database. In their primary paper, they identified that major POCs (requiring re-admission or reoperation) were associated with higher all-cause and cancer-specific mortality rates, especially after mastectomy (all-cause mortality: HR 1.32, 95% CI: 1.15–1.51; cancer-specific mortality: HR 1.31, 95% CI: 1.04–1.65) (7). This study included all patients with primary breast cancer irrespective of nodal status who received breast conservation with radiotherapy, or mastectomy with or without radiotherapy. Patients receiving concurrent breast reconstruction were also included, but only one patient who experienced a POC died within the follow-up period. While this study adjusted for patient demographics, socio-economic deprivation, and tumour characteristics, comorbidities such as body mass index (BMI), smoking status and frailty were not accounted for, all of which are associated with inferior prognosis (39-41). In another paper, de Boniface et al. analysed survival outcomes in women ≥50 years of age. Both major surgical and medical complications were associated with higher all-cause mortality rates in women ≥80 years (major surgical complications: HR 1.43, 95% CI: 1.09–1.86, P=0.009; medical complications: HR 1.60, 95% CI: 1.25–2.06, P<0.001), and major surgical complications were also associated with increased all-cause mortality risk in women aged 50–69 years (7). Cancer-specific mortality was not reported in this paper. The authors did not explain the bimodal distribution, but stressed importance of recommending breast conservation in older women to reduce risk of POCs and increase survival benefit. One other large population study reports an association between POCs and increased risk of all-cause mortality (23). This includes both wound-related and non-wound related complications, similar to the second Swedish paper (34), but only women >65 years were included in this analysis. Women of all ages were included in the primary Swedish paper (7).
Londero et al. assessed survival outcomes in patients who develop a VTE following breast surgery (33). On crude analysis, they demonstrated increased risk of all-cause mortality in patients who experience a thrombotic event within 3 months of breast surgery compared to those who did not. This large study (n=5,039) includes benign breast surgery, and most patients were given thrombotic prophylaxis, which are likely to lower incidence rates. Three smaller studies (n=107–378) did not identify an association between POCs and increased risk of mortality (27,30,31). Two single-limb studies examined outcomes in patients following Mx + IBR (27,31) whilst Hammond et al. included patients undergoing Mx ± IBR in their analysis and came to a similar conclusion (30). Whilst one of these studies has a considerable length of follow-up (106.8 months) (31), 74.1% patients underwent contralateral prophylactic mastectomy. Bilateral surgery increases risk of POCs without expectant inferior oncological outcomes (30). In addition, this study mentions strict selection criteria for immediate reconstruction at their institute. Thus, their cohort represent a ‘relatively healthy’ patient cohort may be less affected by POCs as those in the larger, population databases.
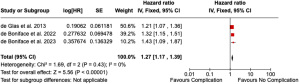
Meta-analysis of three large population studies including 60,331 patients and Newcastle Ottawa Score >8 (7,23,34) demonstrated significant impact of complications after breast cancer surgery on all-cause mortality (HR 1.27, 95% CI: 1.17–1.39, P<0.001). No heterogeneity was observed (I2=0%) (Figure 4).
Discussion
Key findings
This systematic review and meta-analysis demonstrate an association between POCs after breast cancer surgery and inferior oncological outcomes including disease-recurrence and all-cause mortality. The association with all-cause mortality remains strong, however significant heterogeneity in studies means that an association between POCs and disease-recurrence is not clear. Elderly patients particularly have a significantly increased mortality associated with development of a POC. The incidence of POC increases with increasing surgical complexity, with most complications occurring in Mx + IBR, followed by mastectomy alone, followed by conservation surgery. There is conflicting data on whether development of POC does cause a significant delay to adjuvant treatments or not, and whether this correlates to an effect on recurrence and survival.
Strengths and limitations
We systemically reviewed the existing evidence and performed a meta-analysis assessing the association between POCs, disease-recurrence and all-cause mortality in primary breast cancer. This includes the largest available patient samples in the literature with two population-cohort National Cancer Registry studies with long-term follow-up. All studies are, however, observational, retrospective and mostly from a single centre. Undoubtedly, this will result in under-reporting in the incidence of POC. Further limitations of this study include the heterogeneity in POC definitions and reported outcome measures, in particular disease-recurrence sites and variability in the definition of POC between different studies. Consideration of locoregional and systemic disease-recurrence as two separate endpoints will help in understanding the pathophysiology behind any potential association. Only one study reported cancer-specific death independent of all-cause mortality. In addition, there is a paucity of substantive data exploring outcomes following complications after reconstructive and oncoplastic breast cancer surgery, as well as risk assessment for other patient factors such as smoking and obesity.
Comparison with similar research
Two previous systematic reviews have demonstrated similar results for disease-recurrence following POCs, one of which reported an association with disease-free survival (2,19). This is the first study to demonstrate an association between POCs and all-cause mortality.
Explanations of findings
The underlying association between POCs and inferior oncological outcomes is likely due to the synergistic effect of both localized wound healing and systemic inflammation and the post-operative ‘immunosuppression’ window leading to increased growth of occult micro-metastases. Previous reviews have suggested a likely link on a cellular level, and further studies are needed to explore this. There is a clear correlation between the size of surgical insult and the risk of developing a POC with Mx + IBR associated with the highest incidence of POC. Despite this, there is no clear effect on breast cancer recurrence or survival in Mx + IBR patients who do experience a POC. In the largest available population cohort study, there was only 1 death in the group of patients having Mx + IBR despite them having the highest rate of POC of all surgeries. Therefore, they concluded no effect on mortality.
Mx + IBR is a complex surgical intervention and therefore only patients who are fit with no significant comorbidities are deemed appropriate candidates. This selection bias means the average patient with pre-existing health issues and or elderly women will be underrepresented in the Mx + IBR group. This may in part contribute to the low mortality rate seen in this cohort.
Implications and actions needed
Future prospective studies with well-defined inclusion and treatment criteria are required which explore locoregional and systemic recurrence as two separate endpoints, as well as studies exploring cellular level changes in primary breast cancer and its treatment. Oncoplastic conservation surgery is now the standard of care for breast cancer patients. This allows a less invasive surgical approach in larger tumours which traditionally would have required a mastectomy. We know there is a strong correlation between the size of surgical insult and POC. There is, however, a paucity of data on oncoplastic surgery and its associated POC compared to standard conservation surgery and mastectomy with or without reconstruction. It is important to explore this and any association with longer-term oncological outcomes in prospective multi-centre data registries such as the UK ‘PartBreCon’ study (42).
Furthermore, in patients who do require a mastectomy there are numerous surgical reconstruction techniques available with implants placed in the pre-pectoral or sub-pectoral plane with or without use of a mesh or acellular dermal matrix and autologous options involving free tissue transfer. Whilst reconstruction options will be impacted by expertise in particular institutions and individual patient factors, it is crucial that we expose any technique which may be associated with increased risk of POC and adverse outcomes. Reconstruction options are personalized to individual patients making participation in randomized controlled trials not feasible. However, prospective multi-centre data collection should be considered to allow true shared decision-making when counselling patients.
It is clear that the risk of POC correlates to the size of surgical insult and therefore conservation surgery should be the preferred option for all patients where possible, especially in older patients who have a significantly higher mortality with POC.
Conclusions
POCs are associated with increased risk of all-cause mortality after breast cancer surgery, but an association between POC and disease-recurrence is less certain due to heterogeneity.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-24-35/rc
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-24-35/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-24-35/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organisation. Breast Cancer. 2024 [Accessed 2024 Aug 26]. Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer
- O'Connor RÍ, Kiely PA, Dunne CP. The relationship between post-surgery infection and breast cancer recurrence. J Hosp Infect 2020;106:522-35. [Crossref] [PubMed]
- Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers 2019;5:66. [Crossref] [PubMed]
- EBCTCG (Early Breast Cancer Trialists' Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. Erratum in: Lancet 2014;384:1848. [Crossref] [PubMed]
- James Lind Alliance Priority Settings Partnerships. Breast Cancer Surgery Top 10. 2022 [Accessed 2023 Oct 16]. Available online: https://www.jla.nihr.ac.uk/priority-setting-partnerships/breast-cancer-surgery#tab-26461
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- de Boniface J, Szulkin R, Johansson ALV. Major surgical postoperative complications and survival in breast cancer: Swedish population-based register study in 57 152 women. Br J Surg 2022;109:977-83. [Crossref] [PubMed]
- Adwall L, Hultin H, Mani M, et al. Prospective Evaluation of Complications and Associated Risk Factors in Breast Cancer Surgery. J Oncol 2022;2022:6601066. [Crossref] [PubMed]
- Murthy BL, Thomson CS, Dodwell D, et al. Postoperative wound complications and systemic recurrence in breast cancer. Br J Cancer 2007;97:1211-7. [Crossref] [PubMed]
- Beecher SM, O’Leary DP, McLaughlin R, et al. The Impact of Surgical Complications on Cancer Recurrence Rates: A Literature Review. Oncol Res Treat 2018;41:478-82. [Crossref] [PubMed]
- van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Kubota T, Hiki N, Sano T, et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol 2014;21:891-8. [Crossref] [PubMed]
- Andalib A, Ramana-Kumar AV, Bartlett G, et al. Influence of postoperative infectious complications on long-term survival of lung cancer patients: a population-based cohort study. J Thorac Oncol 2013;8:554-61. [Crossref] [PubMed]
- Boukovalas S, Goepfert RP, Smith JM, et al. Association between postoperative complications and long-term oncologic outcomes following total laryngectomy: 10-year experience at MD Anderson Cancer Center. Cancer 2020;126:4905-16. [Crossref] [PubMed]
- Atsumi Y, Aoyama T, Tamagawa A, et al. Association between postoperative pneumonia and prognosis of patients with esophageal cancer. J Clin Oncol 2020;38:370. [Crossref]
- Tang F, Tie Y, Tu C, et al. Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies. Clin Transl Med 2020;10:199-223. [Crossref] [PubMed]
- van der Bij GJ, Oosterling SJ, Beelen RH, et al. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg 2009;249:727-34. [Crossref] [PubMed]
- Dvorak HF. Tumors: Wounds That Do Not Heal. N Engl J Med 1986;315:1650-9. [PubMed]
- Savioli F, Edwards J, McMillan D, et al. The effect of postoperative complications on survival and recurrence after surgery for breast cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2020;155:103075. [Crossref] [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000 [Accessed 2024 Sept 10]. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Indelicato D, Grobmyer SR, Newlin H, et al. Association between operative closure type and acute infection, local recurrence, and disease surveillance in patients undergoing breast conserving therapy for early-stage breast cancer. Surgery 2007;141:645-53. [Crossref] [PubMed]
- Yan T, Yin W, Zhou L, et al. Postoperative fever: the potential relationship with prognosis in node negative breast cancer patients. PLoS One 2010;5:e15903. [Crossref] [PubMed]
- de Glas NA, Kiderlen M, Bastiaannet E, et al. Postoperative complications and survival of elderly breast cancer patients: a FOCUS study analysis. Breast Cancer Res Treat 2013;138:561-9. [Crossref] [PubMed]
- Beecher SM, O'Leary DP, McLaughlin R, et al. Influence of complications following immediate breast reconstruction on breast cancer recurrence rates. Br J Surg 2016;103:391-8. [Crossref] [PubMed]
- Pedersen RN, Bhaskaran K, Heide-Jørgensen U, et al. Breast cancer recurrence after reoperation for surgical bleeding. Br J Surg 2017;104:1665-74. [Crossref] [PubMed]
- Mousa M, Barnea Y, Arad U, et al. Association Between Postoperative Complications After Immediate Alloplastic Breast Reconstruction and Oncologic Outcome. Clin Breast Cancer 2018;18:e699-702. [Crossref] [PubMed]
- Abdullah N, O'Leary DP, Hegarty A, et al. The effect of surgical site infection in immediate breast reconstruction on breast cancer recurrence. Breast J 2019;25:166-8. [Crossref] [PubMed]
- Valente SA, Liu Y, Upadhyaya S, et al. The effect of wound complications following mastectomy with immediate reconstruction on breast cancer recurrence. Am J Surg 2019;217:514-8. [Crossref] [PubMed]
- Lee KT, Jung JH, Mun GH, et al. Influence of complications following total mastectomy and immediate reconstruction on breast cancer recurrence. Br J Surg 2020;107:1154-62. [Crossref] [PubMed]
- Hammond JB, Han GR, Cronin PA, et al. Exploring the Effect of Post-mastectomy complications on 5-year survival. Am J Surg 2020;220:1422-7. [Crossref] [PubMed]
- Siegel EL, Whiting J, Kim Y, et al. Effect of surgical complications on outcomes in breast cancer patients treated with mastectomy and immediate reconstruction. Breast Cancer Res Treat 2021;188:641-8. [Crossref] [PubMed]
- Adwall L, Pantiora E, Hultin H, et al. Association of postoperative infection and oncological outcome after breast cancer surgery. BJS Open 2021;5:zrab052. [Crossref] [PubMed]
- Londero AP, Bertozzi S, Cedolini C, et al. Incidence and Risk Factors for Venous Thromboembolism in Female Patients Undergoing Breast Surgery. Cancers (Basel) 2022;14:988. [Crossref] [PubMed]
- de Boniface J, Szulkin R, Johansson ALV. Medical and surgical postoperative complications after breast conservation versus mastectomy in older women with breast cancer: Swedish population-based register study of 34 139 women. Br J Surg 2023;110:344-52. [Crossref] [PubMed]
- B Jackson E. Does age affect outcome with breast cancer? Breast 2023;70:25-31. [Crossref] [PubMed]
- Bennett KG, Qi J, Kim HM, et al. Comparison of 2-Year Complication Rates Among Common Techniques for Postmastectomy Breast Reconstruction. JAMA Surg 2018;153:901-8. [Crossref] [PubMed]
- Antram E, Shaari E, Balasubramanian R, et al. Investigating the time to adjuvant treatment following immediate breast reconstruction in breast cancer patients. Ann Breast Surg 2023;7:15. [Crossref]
- van Maaren MC, de Munck L, Strobbe LJA, et al. Ten-year recurrence rates for breast cancer subtypes in the Netherlands: A large population-based study. Int J Cancer 2019;144:263-72. [Crossref] [PubMed]
- Sun L, Zhu Y, Qian Q, et al. Body mass index and prognosis of breast cancer. Medicine (Baltimore) 2018;97:e11220. [Crossref] [PubMed]
- Pierce JP, Patterson RE, Senger CM, et al. Lifetime cigarette smoking and breast cancer prognosis in the After Breast Cancer Pooling Project. J Natl Cancer Inst 2014;106:djt359. [Crossref] [PubMed]
- Coleman C, Yan CH, Ko NY, et al. Physical functioning, frailty and risks of locally-advanced breast cancer among older women. Breast 2022;64:19-28. [Crossref] [PubMed]
- Agrawal A, Romics L, Thekkinkattil D, et al. 'PartBreCon' study. A UK multicentre retrospective cohort study to assess outcomes following PARTial BREast reCONstruction with chest wall perforator flaps. Breast 2023;71:82-8. [Crossref] [PubMed]
Cite this article as: Mactier M, Arthur L, Edwards J, Doughty J, Romics L. Exploring the association between post-operative complications, disease-recurrence & mortality in primary operable breast cancer—systematic review & meta-analysis. Ann Breast Surg 2025;9:12.


