
Current practices regarding pre-pectoral breast reconstruction following mastectomy for breast cancer: an ASBrS survey gauging support for a national registry
Highlight box
Key findings
• Surgeons disagree on how to surveil patients with pre-pectoral breast reconstruction (PPBR). Most respondents agree that a national registry would be beneficial.
What is known and what is new?
• PPBR is repopularizing due to factors such as improved cosmesis and pain post-operatively.
• There is no clear consensus regarding how to surveil these patients and whether physical exam alone versus imaging is adequate.
What is the implication, and what should change now?
• Surveyed surgeons support that the creation of a national registry is indicated to guide future surveillance of cancer recurrence in patients with PPBR.
Introduction
Breast cancer is the most common cancer among women in the U.S. and the second leading cause of cancer-related mortality among women (1). Breast reconstruction following mastectomy is commonly offered to breast cancer patients and is an area of considerable research and development. The American Society of Plastic Surgeons reports that in 2018, over 101,000 reconstructions were performed across the many different techniques. About 83,000 involved implants, with 70,000 of these involving a tissue expander and/or implant, about 9,500 involved a deep inferior epigastric perforator (DIEP) flap, and the remaining 3,700 implants utilized a form of transverse rectus abdominis myocutaneous (TRAM) flap (2). The pre-pectoral breast reconstruction (PPBR) approach, initially utilized in the 1990s, has regained popularity and use in these patients over the traditional subpectoral breast reconstruction (SBR) approach (3,4). In PPBR, the implant is placed superficial to the pectoralis major muscle, while it is placed deep to the muscle in SBR. The benefits of PPBR over SBR include reduced post-operative pain from displacing the pectoralis muscle and in turn decreased length of stay (LOS), reduced animation deformity, and overall greater patient satisfaction with cosmetic outcomes (5-7). Additionally, the implementation of human acellular dermal matrices (ADMs) for PPBR implants reduces capsular contraction rates (8). While one meta-analysis by Nolan et al. (9) found that patients with PPBR have significantly lower rates of mastectomy flap necrosis compared to SBR, other studies have found no significant differences in the rates of flap necrosis, implant loss, hematoma, seroma, surgical site infection or reoperation rates (10). It is worth noting, however, that while implant loss is reportedly lower in PPBR compared to SBR (10), reconstructive failure as a result of implant exposure is still a significant risk with PPBR, affecting 6.8% of reconstructed breasts in a prospective cohort study by di Pompeo (11), comparable to 6.5% reported in Masià’s (12) iBAG (international Braxon Audit Group) study on PPBRs.
Numerous studies, including a systematic review and meta-analysis by Li et al. (10), have shown there to be no statistically significant difference in locoregional recurrence (LRR) rates between SBR and PPBR cohorts. However, specific data regarding time to and detection of recurrence after PPBR are lacking, likely due to the recent repopularization of this technique. Much of the data that can be found are only complementary in nature to another primary focus without in-depth insight into the matter, small-scale in nature, or are retrospective cohort studies where data are obtained from date ranges that precede PPBR’s re-emergence.
Moreover, there is no widely accepted surveillance protocol established for these patients, as the data needed to inform guidelines are limited. Some papers have noted concern regarding whether the superficial implant placement in PPBR might interfere with the ability to detect recurrent cancer in the underlying chest wall on physical exam, though it is unclear if data support this assumption (10).
In this study, we aim to utilize a survey of breast surgeons to determine their familiarity with this resurgent, more widely adopted reconstruction technique, inquire about concerns regarding determining cancer recurrence, surveillance imaging, and gauge interest in a national registry to begin gathering data on this subset of patients. We present this article in accordance with the SURGE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-24-58/rc).
Methods
A twelve-question unincentivized survey (Table 1), along with a brief description of the purpose of the study, was sent via email once to all members of the American Society of Breast Surgeons (ASBrS), an organization comprised of general surgeons who treat patients with breast disease. The survey was emailed to 2,927 members, of which 416 responded for a response rate of 14.21%. As the organization includes both academic and community setting surgeons, the sample represents all settings of breast care. The survey examined knowledge/familiarity of PPBR, concern regarding the ability to detect chest wall recurrence, need for routine surveillance, and support for the creation of a national registry. Survey questions were conceptualized and developed with a committee of breast surgeons.
Table 1
| # | Question | Response | N | % | Total N |
|---|---|---|---|---|---|
| 1 | Are you an active practicing surgeon that performs breast surgery? | Yes | 406 | 97.8 | 415 |
| No | 9 | 2.2 | |||
| 2 | In the last year, what percentage of your surgeries are breast surgeries (non-plastic/reconstruction)? | <10% | 6 | 1.5 | 413 |
| 10–24% | 21 | 5.1 | |||
| 25–49% | 37 | 9.0 | |||
| 50–75% | 37 | 9.0 | |||
| >75% | 312 | 75.5 | |||
| 3 | Do you consider the majority of your current practice to be academic-based or private practice? | Academic | 121 | 29.2 | 415 |
| Private | 240 | 57.8 | |||
| Other | 54 | 13.0 | |||
| 4 | Do you work with a plastic surgeon who has performed pre-pectoral implant/tissue expander placement after mastectomies on your patient? | Yes | 378 | 90.9 | 416 |
| No | 34 | 8.2 | |||
| I don’t know | 4 | 1.0 | |||
| 5 | If you work with a plastic reconstructive surgeon, within the last year, how many cases on average were performed with pre-pectoral implant/tissue expander placement after mastectomy on your patients? | 0 | 37 | 9.0 | 410 |
| <10 | 100 | 24.0 | |||
| 10–19 | 87 | 21.2 | |||
| 20–30 | 67 | 16.3 | |||
| >30 | 107 | 26.1 | |||
| I don’t know | 12 | 2.9 | |||
| 6 | For your patients who had a unilateral or bilateral mastectomy with or without reconstruction, do you (the surgeon) continue to follow your patients for ongoing oncologic surveillance for at least two years? | Yes, more than two years total | 356 | 86.0 | 414 |
| Yes, but less than two years total | 34 | 8.2 | |||
| No, I don’t routinely follow my patients beyond their post-operative check for ongoing oncologic surveillance | 24 | 5.8 | |||
| 7 | Do you ever order any routine annual surveillance imaging (i.e., breast MRI, PET/CT, CT scans, ultrasounds, etc.) on your breast cancer patients (stage 0–stage III) who have had unilateral or bilateral mastectomies without reconstruction for continued evaluation of the affected side? | Yes | 46 | 11.1 | 413 |
| No | 367 | 88.9 | |||
| 8 | Do you ever order any routine annual surveillance imaging (i.e., breast MRI, PET/CT, CT scans, ultrasounds, etc.) on your breast cancer patients (stage 0–stage III) who have had unilateral or bilateral mastectomies with reconstruction for continued evaluation of the affected side? | Yes | 82 | 19.9 | 413 |
| No | 331 | 80.1 | |||
| 9 | Do you feel the physical exam for detecting recurrence is limited due to the implant location for these patients? | Yes | 140 | 34.0 | 412 |
| No | 215 | 52.2 | |||
| I don’t know | 57 | 13.8 | |||
| 10 | Do you think it would be helpful to aid in detecting recurrence by acquiring imaging to supplement the physical exam for this specific subset of patients? | Yes | 142 | 34.5 | 411 |
| No | 171 | 41.6 | |||
| I don’t know | 98 | 23.8 | |||
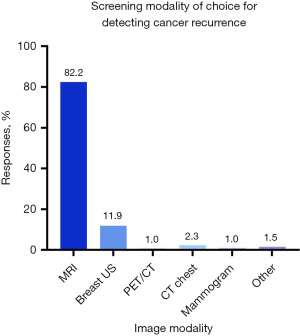
| 11 | If additional imaging were ordered to evaluate for recurrence in patients undergoing pre-pectoral implant reconstruction, which imaging modality do you believe would be most appropriate/helpful for annual oncologic surveillance of local recurrence? | Breast MRI | 319 | 82.2 | 388 |
| Breast US | 46 | 11.9 | |||
| CT chest | 9 | 2.3 | |||
| PET/CT | 4 | 1.0 | |||
| Mammograms | 4 | 1.0 | |||
| Other | 6 | 1.5 | |||
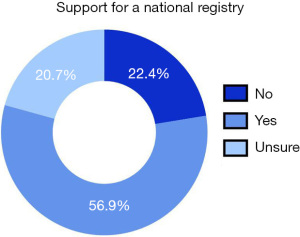
| 12 | Should patients with a history of breast cancer (who undergo unilateral or bilateral mastectomy with pre-pectoral (on top of the muscle) reconstruction with permanent implants) be tracked via a national registry/database for recurrence? | Yes | 236 | 56.9 | 415 |
| No | 93 | 22.4 | |||
| I don’t know | 86 | 20.7 |
CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; US, ultrasound.
Statistical analysis
A minimum sample size of 350 was calculated for the 95% confidence interval, given the number of members who received the emailed survey. The large sample size, clear informed consent, and survey administration via a large listserv with a broad array of practice types served to reduce selection bias. SPSS 29.0 was used to perform descriptive analysis and Chi-squared tests with the level of significance set at 0.05. Figures were created in GraphPad Prism 10. Analysis and reporting were conducted in accordance with statistical analyses and methods in the published literature (SAMPL) guidelines. Missing data were handled question by question.
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. No patients were involved in this study, and approval from the Ethics Committee was not necessary. Participation in this survey was voluntary and anonymous, and informed consent was obtained from all participants.
Results
A total of 416 surgeons responded to the survey, with 406 respondents being active, practicing breast surgeons (Table 1). Most respondents (75.5%) reported that >75% of their practice is breast surgery (Table 1). Private practice was the most common practice type, making up 57.8% of responses, with academic practice representing 29.2% (Table 1). Ninety-one percent of breast surgeons reported working with a plastic surgeon who performs PPBR on their patients following mastectomy (Table 1). Twenty-six percent of respondents indicated that on average, over 30 of their cases were performed with pre-pectoral implant/tissue expander placement after mastectomy, while 16.3% reported 20–30 cases, 21.2% reported 10–19 cases, 24.0% reported less than 10 cases, and 9.0% reported 0 cases Respondents who reported performing 0 or <10 PPBC in the last year reported the lowest trust in physical exam for cancer recurrence (42.9% and 50.0%, respectively, reported the physical exam to be limited) (Table 1). Respondents who perform 10–19, 20–30, and >30 PPBC per year less frequently reported the physical exam to be limited (27.9%, 35.8%, 20.6%, respectively) (Table 1). Statistical analysis found the number of PPBC cases performed to be significantly associated with trust in the physical exam (P<0.001).
Most respondents (52.2%) do not feel like the physical exam is limited in PPBR patients, while 34.0% do perceive the physical exam to be limited, and 13.8% are unsure (Figure 1). The private practice surgeons were more likely to find the physical exam limited for detection of cancer recurrence (45.1%) compared to academic surgeons (18.2%), with analysis showing a statistically significant association between practice type and trust in physical exam (P<0.001).
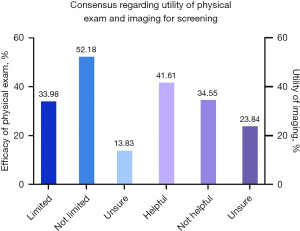
A slight majority of respondents (41.6%) do not believe that imaging would be a helpful adjunct to physical exam in detecting cancer recurrence in PPBR patients, while 34.5% believe it would be helpful, and 23.8% are unsure (Figure 1). If additional imaging were to be used to monitor for surveillance, most respondents (82.2%) believe magnetic resonance imaging (MRI) would be most appropriate, followed by ultrasound (11.9%), computed tomography (CT) chest (2.3%), positron emission tomography (PET)/CT (1.0%), and mammogram (1.0%). Some respondents chose other for this question and suggested the use of an abbreviated MRI (Figure 2).
Of the 34.0% of respondents who reported that the physical exam is limited in detecting recurrence in PPBR patients, 70.7% supported the use of supplemental imaging, while 10.0% did not believe supplemental imaging is helpful, and 19.3% were unsure. Of the 52.2% of respondents who reported trust in the physical exam, 70.1% did not believe supplemental imaging was helpful, 11.7% believed it would be helpful, and 18.2% were unsure. Statistical analysis found trust in physical exam is significantly associated with support for supplemental imaging (P<0.001).
Overall, 94.2% of survey respondents follow patients after mastectomy, despite whether they did or did not receive any reconstruction (Table 1). Most of these respondents follow patients for at least 2 years (86.0%), with only 8.2% following patients for less than 2 years (Table 1). Most respondents (88.9%) do not order routine surveillance imaging for patients who undergo mastectomy without reconstruction for stage 0–III disease (Table 1). A slightly smaller proportion (80.1%) do not order routine surveillance for patients who do receive reconstruction (Table 1).
Only 22.4% of surgeons did not feel a national registry was needed to track cancer patients who have undergone PPBR, whereas 56.9% of surgeons agreed that a registry was needed, and 20.7% were unsure (Figure 3).
Discussion
With the resurgence of PPBR, it is paramount that surveillance techniques and guidelines are optimized to detect cancer recurrence in this patient population. A meta-analysis published by Ostapenko et al. (7) notes that PPBRs have a cancer recurrence rate of 2.77%, while SBRs have recurrence rates of 1.91%, though notably no statistically significant difference was appreciated. It should also be noted that the authors acknowledge that the shorter follow-up times in the PPBR group compared to the SBR group hinder the ability to assess long-term oncologic outcomes (7). It is important to consider that individuals with a recurrence may have a distant recurrence, possibly making the incidence within local tissues less relevant to overall outcomes. A single institution case-control study by Sosin et al. (13) broadly focusing on all implant types in prior breast augmentation found that the mode of initial cancer detection was palpation of a mass in 57.1% of PPBR and 54.8% of SBR patients. Mammogram was the mode of detection in 41.9% of SBR versus 28.6% in PPBR (13). Additionally, 14% of those with PPBR implants were initially detected by MRI, although there may have been selection bias because a higher number of the PPBR cohort were BRCA mutation carriers with indication for MRI surveillance (13). It should be noted that the patients in the Sosin study still have breast tissue as they underwent breast augmentation and not mastectomy for oncologic treatment. Although we are evaluating the surveillance for cancer recurrence in post-mastectomy patients who presumably do not have breast tissue remaining, the results of the Sosin study still serve to quantify detection based on reconstructive technique. Mirzabeigi et al. (14) also performed a retrospective cohort study examining LRR in women post-reconstruction from records dating January 2010 to July 2014. Importantly, the study showed that a self-breast exam (SBE) was the most likely means of initial detection. The LRR locations for implant-based reconstruction in this study were subcutaneous (27%), subcutaneous/pectoralis muscle (24%), chest wall (37%), and axilla (12%) (14). As the study was presumptively done before the implementation of PPBR reconstruction, the results are likely not directly generalizable to patients with PPBR. However, considering 35% of implant-based recurrences are in the chest wall, the presence of an implant overlying the pectoralis, such as in PPBR, inherently raises concern that recurrences may be missed. Conversely, a subpectoral implant would keep the anterior pectoralis flush with overlying skin, making palpation as a means for detecting a new mass potentially more sensitive. It is also important to consider that recurrence in the subcutaneous space may still be superficial to the pre-pectoral implant based on the depth of the subcutaneous pocket. This would still allow for palpation of the subcutaneous space against the implant provided minimal subcutaneous tissue is present between the implant and pectoralis muscle. This, of course, would be limited in cases with involvement of the pectoralis muscle which would still be deep to the implant.
Autologous reconstructions inhabit similar anatomic spaces as implants and provide added insight for common LRR locations to help determine the ideal surveillance method for timely detection. The study by Mirzabeigi et al. (14) found that for TRAM/DIEP flap reconstruction, LRR was in the subcutaneous space in 47% and the chest wall in 41%. Compared to implant-based reconstructions, TRAM/DIEP flaps exhibited a lower likelihood of index reconstruction failure in patients with cancer recurrence (14). Paradoxically, this study also found that autologous reconstructions were more likely to present with advanced-stage recurrent cancer compared to those who had implant reconstruction (14). Of note, the average LRR size was 1.5 cm in the implant reconstruction group compared to 2.9 cm in TRAM/DIEP reconstruction (14). This is concerning as TRAM/DIEP reconstruction could theoretically pose a similar barrier to detecting a mass in the subcutaneous/pectoralis plane or chest wall that a pre-pectoral implant could create and may therefore likewise benefit from a national registry (14). Fat grafts are used for total reconstruction or added contour refinement following implant placement and do not show higher rates of LRR compared to mastectomy without reconstruction, but could similarly impede early detection (15). Such potential barriers would be important to recognize given that patients and providers may favor autologous reconstruction due to concern for Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) as well as desire for increased satisfaction with the operation (16,17). Conversely, physicians from our study and others may opt for implant reconstruction to avoid the higher risk for thromboembolic events with prolonged autologous reconstruction operation times (18,19). A 2010 study by Zakhireh et al. (20) suggests recurrence following autologous flap reconstruction may be difficult to detect by clinical breast exam (CBE) if the tumor occurs along the posterior margin behind the flap. However, with only soft tissue present, these patients’ chest wall can be examined readily with ultrasound and/or mammogram if a suspicious finding is present during physical exam. Given the theoretical similarities in clinical challenges between PPBR and autologous flap reconstruction, Sigalove et al. (3) proposed to employ a similar protocol as that used for women following autologous reconstruction. This protocol generally entails biannual CBE and monthly SBE. They highlight that mammography is of limited benefit for detecting LRR in autologous reconstruction and thus advise against routine use of mammography for PPBR. National guidelines reflect this ideology as the National Comprehensive Cancer Network (NCCN) recommends against routine imaging following mastectomy with or without reconstruction, and the American College of Radiology does not recommend mammography or digital breast tomosynthesis in mastectomy with implant reconstruction. The Food and Drug Administration (FDA) recommends routine implant surveillance in all women (following cosmetic and/or reconstruction surgery), employing ultrasound or MRI at post-operative year 5–6, then every 2 years afterwards, which serves to monitor for implant integrity and cancer recurrence (21). While routine imaging for breast implants is recommended by the FDA, the lack of standardized guidelines specifically for PPBR patients may contribute to the low compliance among surgeons to implement routine imaging. In addition, concerns regarding the efficacy of physical exams and the potential limitations of imaging modalities for detecting chest wall recurrence further complicate the decision to implement routine surveillance imaging. However, considering that recurrence can occur distally, the effectiveness of routine surveillance in improving overall survival should be properly assessed and understood. This FDA recommendation is likely reflected in the rates of follow-up demonstrated in this study that show most breast surgeons surveyed follow patients for at least 2 years post-operatively. However, the study by Lee et al. (22) discussed that LRR usually occurs within the first 3 years post-operatively, peaking in the 2nd year, which we propose shows that delaying MRI until the 5th–6th year post-operatively may allow for disease progression. Given the frequency with which cancer recurrence is detected with SBE, the 2022 Getting It Right First Time (GIRFT) report from the UK recommends a “patient-initiated follow-up” strategy in place of routine physical exams after mastectomy and reconstruction, with no evidence of an increase in local recurrence rates since it was adopted in 2017/2018 (23). This patient-driven surveillance approach could offer insights for PPBR patients, especially since many surgeons in our study expressed uncertainty about the limitations of physical exams in detecting recurrence.
Because of the unique placement of pre-pectoral implants, patients who undergo this procedure following mastectomy for breast cancer may benefit from more tailored imaging to screen for recurrence. The American College of Surgeons (ACS) currently reserves screening breast MRI as a supplement to mammograms in high-risk women, but its sensitivity for detecting lesions likely explains why most respondents to our survey considered MRI the most appropriate annual oncologic surveillance of local recurrence for post-mastectomy PPBR. MRI may be favored over mammography in this population due to the absence of breast tissue. While MRI has a higher sensitivity for detecting recurrence, utilizing it as a screening modality is limited by the duration and cost of the exam. Newer radiologic techniques, such as the abbreviated and ultrafast breast MRI, may offer the perfect balance of sensitivity and feasibility.
The abbreviated MRI shortens the standard breast MRI protocol to less than 10 minutes and can be achieved with standard MRI devices. Importantly, the abbreviated MRI shows similar sensitivity for detecting cancers as the conventional MRI protocol. Ultrafast MRI works even faster by imaging within the first two minutes of contrast injection, but is limited by the more elaborate technique employed to obtain images: the requirement of a specific coil and sequence-type means the ultrafast MRI cannot be employed with most commercially available units (24). In a recent study of 867 women with a personal history of breast cancer, abbreviated MRI had a sensitivity of 90% and specificity of 88.6%, demonstrating validity in women with previous breast cancer who are not necessarily high risk (25). The specificity was further increased to 95.3% in combination with ultrafast MRI in this population (25). While abbreviated and ultrafast MRI techniques have limitations, such as the use of contrast and lower specificity, they could still be an important aid in screening for local recurrence following PPBR after mastectomy.
Since abbreviated and ultrafast MRI is not used routinely for breast imaging, it is not known yet whether a different surveillance method would be more cost-effective. Abbreviated MRI is estimated to have a 79% chance of being a cost-effective alternative to mammography among women aged 50–65 with extremely dense breasts (26). It was not suggested to be cost-effective with heterogeneously dense breasts. Breast implants are not considered dense and may not benefit from a similar cost-effectiveness based on this data. However, compared to digital breast tomosynthesis, abbreviated MRI shows improved cost effectiveness in people with intermediate risk for breast cancer, which could benefit those who require double mastectomy (27).
Given that one component of the criteria for breast center designation is >150 annual cases, it is likely that a portion of our survey respondents do not work at a designated breast center, as 9% of our respondents perform <10 PPBR surgeries annually (28). However, it should be noted that designation is based on total center case volume, not per surgeon volume, as our study represents. The difference between the trust in the physical exam for academic versus private surgeons is interesting, and though there are no studies explaining why this phenomenon exists, the authors postulate that resource allocation may play a role. Our survey also did not evaluate for dual-trained ASBrS members who may perform both mastectomy and reconstruction (29). Such practices are more common in Europe than in the US, though investigation into the practice pattern of this demographic may prove insightful given their involvement in oncologic and reconstructive care.
The results of this study represent the general attitudes of practicing breast surgeons in the United States. As the first study to investigate the re-popularization of PPBR and potential discord among breast surgeons regarding surveillance, the results of this study will lead to the creation of a national registry to further investigate the need for surveillance in this patient population. Such a registry will supplement pre-existing registries such as the Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology (PROFILE) and the Federal Drug Administration Manufacturer and User Facility Device Experience (FDA MAUDE), which do not track recurrence of breast cancer and do not differentiate between different layers of implant placement (30). While the National Breast Implant Registry (NBIR) collects information on implant placement location and reasons for re-operation following implant placement, it does not report on which surveillance method identifies LRR, prompting re-operation (31). It is unclear how surgeons typically follow up patients with mastectomy with or without reconstruction, though patients with reconstruction are more likely to be imaged by non-surgical physicians (32). A national registry would provide data to construct evidence-based guidelines for this population that is currently managed largely experientially. Evidence-based guidelines promote a consistent follow-up approach for surgeons to optimize the chances of early breast cancer recurrence detection. A national registry would have some drawbacks, including a delay in gathering data, especially given the narrow scope of this population. Registries also must be appropriately funded, with special attention paid to the input of pertinent and inclusive data from providers, either through the institutional electronic health record or manual input. Despite the challenges involved in the creation of a national registry, it is still paramount to confirm that local recurrence is not underestimated with current surveillance guidelines.
Limitations
Although the study had an adequate sample size for statistical analysis, a greater number of respondents would have strengthened the assertions of the study. Since the survey was distributed via email, there is no way to ascertain how many members saw the survey and decided not to participate, therefore limiting our ability to more accurately determine the denominator for respondent numbers. Additional techniques (e.g., random sampling, diverse recruitment modalities) aimed at reducing selection bias would also benefit the study. Further analyses could have been applied to the survey data if answers had not been multiple choice. Allowing for non-categorical numerical answers could provide more detail regarding the number of post-mastectomy PPBR operations surgeons are involved in or the frequency of surveillance imaging. Allowing for open-ended answers to questions about whether physicians rely more on physical exam or imaging for the detection of local recurrence would have allowed for a qualitative analysis investigating the reasoning behind these decisions. Additionally, questions addressing demographics, geographics, and institutional resources would strengthen the research and provide a more robust analysis. Furthermore, it would be helpful to understand which kind of surveillance imaging surgeons are ordering on this subset of patients and if it has been successful in detecting recurrence. Further research to determine a “number needed to treat” would be instrumental in determining a best practice for implant screening, and the registry could serve as a data source for this analysis.
Conclusions
The resurgence of PPBR is evident among plastic surgeons due to improved cosmesis, reduced pain, shorter operative times, and shorter hospital stays. Our survey among breast surgeons allowed insight into familiarity with this technique, concerns about detecting recurrence, and the variability in the mode and frequency of post-reconstructive surveillance, if indicated at all. The survey indicates a lack of consensus regarding the utility in physical exams alone versus supplemental imaging in detecting recurrence in those undergoing PPBR. Moreover, the survey conveys support by breast surgeons for the creation of a national registry to track this subset of patients for the location of recurrence and mode of detection. Information gained from the registry will aid in the development of national guidelines to provide more cohesive standards for routine surveillance.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the SURGE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-24-58/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-24-58/dss
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-24-58/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-24-58/coif). Alicia Arnold reports receiving CME/travel reimbursement from her institution, the Medical College of Georgia. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Steiner E, Austin DF, Prouser NC. Detection and description of small breast masses by residents trained using a standardized clinical breast exam curriculum. J Gen Intern Med 2008;23:129-34. [Crossref] [PubMed]
- Plastic Surgery Statistics Report [Internet]. 2018. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Rebowe RE, Allred LJ, Nahabedian MY. The Evolution from Subcutaneous to Prepectoral Prosthetic Breast Reconstruction. Plast Reconstr Surg Glob Open 2018;6:e1797. [Crossref] [PubMed]
- Antony AK, Robinson EC. An Algorithmic Approach to Prepectoral Direct-to-Implant Breast Reconstruction: Version 2.0. Plast Reconstr Surg 2019;143:1311-9. [Crossref] [PubMed]
- Cattelani L, Polotto S. The Economics of Prepectoral Breast Reconstruction. Plast Reconstr Surg 2018;142:415e-7e. [Crossref] [PubMed]
- Ostapenko E, Nixdorf L, Devyatko Y, et al. Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction: A Systemic Review and Meta-analysis. Ann Surg Oncol 2023;30:126-36. [Crossref] [PubMed]
- Mangialardi ML, Salgarello M, Cacciatore P, et al. Complication Rate of Prepectoral Implant-based Breast Reconstruction Using Human Acellular Dermal Matrices. Plast Reconstr Surg Glob Open 2020;8:e3235. [Crossref] [PubMed]
- Nolan IT, Farajzadeh MM, Bekisz JM, et al. Prepectoral versus Subpectoral Breast Reconstruction after Nipple-sparing Mastectomy: A Systematic Review and Meta-Analysis. Plast Reconstr Surg Glob Open 2024;12:e5808. [Crossref] [PubMed]
- Li L, Su Y, Xiu B, et al. Comparison of prepectoral and subpectoral breast reconstruction after mastectomies: A systematic review and meta analysis. Eur J Surg Oncol 2019;45:1542-50. [Crossref] [PubMed]
- di Pompeo FS, Firmani G, Paolini G, et al. Immediate prepectoral breast reconstruction using an ADM with smooth round implants: A prospective observational cohort study. J Plast Reconstr Aesthet Surg 2023;80:56-65. [Crossref] [PubMed]
- Masià J. iBAG Working Group. The largest multicentre data collection on prepectoral breast reconstruction: The iBAG study. J Surg Oncol 2020;122:848-60. [Crossref] [PubMed]
- Sosin M, Devulapalli C, Fehring C, et al. Breast Cancer following Augmentation Mammaplasty: A Case-Control Study. Plast Reconstr Surg 2018;141:833-40. [Crossref] [PubMed]
- Mirzabeigi MN, Rhemtulla IA, Mcdonald ES, et al. Locoregional Cancer Recurrence after Breast Reconstruction: Detection, Management, and Secondary Reconstructive Strategies. Plast Reconstr Surg 2019;143:1322-30. [Crossref] [PubMed]
- Sorotos M, Paolini G, D'Orsi G, et al. Long-Term Clinical and Aesthetic Results of a Systematic Fat Transfer Protocol for Total Breast Reconstruction after Nipple-Sparing Mastectomy. Plast Reconstr Surg 2022;150:5-15. [Crossref] [PubMed]
- Santanellidi Pompeo F, Firmani G, Tornambene R, et al. The impact of Breast Implant-Associated Anaplastic Large Cell Lymphoma on breast implant surgery: A European survey-based study. J Plast Reconstr Aesthet Surg 2025;100:219-30. [Crossref] [PubMed]
- Persichetti P, Barone M, Salzillo R, et al. Impact on Patient's Appearance Perception of Autologous and Implant Based Breast Reconstruction Following Mastectomy Using BREAST-Q. Aesthetic Plast Surg 2022;46:1153-63. [Crossref] [PubMed]
- Paolini G, Firmani G, Sorotos M, et al. European guidelines on peri-operative venous thromboembolism prophylaxis: first update.: Chapter 8: Plastic surgery. Eur J Anaesthesiol 2024;41:598-603. [Crossref] [PubMed]
- Santanelli di Pompeo F, Paolini G, D'Orsi G, et al. Free-style technique versus computed tomographic angiography-guided perforator selection in deep inferior epigastric perforator flap harvest: A prospective clinical study. Microsurgery 2023;43:790-9. [Crossref] [PubMed]
- Zakhireh J, Fowble B, Esserman LJ. Application of screening principles to the reconstructed breast. J Clin Oncol 2010;28:173-80. [Crossref] [PubMed]
- Recommended Follow-Up for Women with Breast Implants. US Food and Drug Administration; 2019.
- Lee SB, Lee JW, Kim HJ, et al. Long-term outcomes of patients with breast cancer after nipple-sparing mastectomy/skin-sparing mastectomy followed by immediate transverse rectus abdominis musculocutaneous flap reconstruction: Comparison with conventional mastectomy in a single center study. Medicine (Baltimore) 2018;97:e0680. [Crossref] [PubMed]
- MacNeill F, Irvine T. Breast Surgery: GIRFT Programme National Specialty Report. Getting It Right First Time; 2021:36.
- Gao Y, Heller SL. Abbreviated and Ultrafast Breast MRI in Clinical Practice. Radiographics 2020;40:1507-27. [Crossref] [PubMed]
- Kim ES, Cho N, Kim SY, et al. Added value of ultrafast sequence in abbreviated breast MRI surveillance in women with a personal history of breast cancer: A multireader study. Eur J Radiol 2022;151:110322. [Crossref] [PubMed]
- Wang J, Greuter MJW, Vermeulen KM, et al. Cost-effectiveness of abbreviated-protocol MRI screening for women with mammographically dense breasts in a national breast cancer screening program. Breast 2022;61:58-65. [Crossref] [PubMed]
- Tollens F, Baltzer PAT, Dietzel M, et al. Cost-Effectiveness of Digital Breast Tomosynthesis vs. Abbreviated Breast MRI for Screening Women with Intermediate Risk of Breast Cancer-How Low-Cost Must MRI Be? Cancers (Basel) 2021;13:1241. [Crossref] [PubMed]
- Biganzoli L, Cardoso F, Beishon M, et al. The requirements of a specialist breast centre. Breast 2020;51:65-84. [Crossref] [PubMed]
- Gemignani ML, Disa JJ. Discussion: The Impact of a Single Dual-Trained Surgeon in the Management of Mastectomy and Reconstruction. Plast Reconstr Surg 2022;149:829-30. [Crossref] [PubMed]
- Santanelli di Pompeo F, Clemens MW, Atlan M, et al. 2022 Practice Recommendation Updates From the World Consensus Conference on BIA-ALCL. Aesthet Surg J 2022;42:1262-78. [Crossref] [PubMed]
- National Breast Implant Registry (NBIR) Annual Report 2021. The Plastic Surgery Foundation; 2021.
- Shammas RL, Broadwater G, Cason RW, et al. Assessing the Utility of Post-Mastectomy Imaging after Breast Reconstruction. J Am Coll Surg 2020;230:605-614.e1. [Crossref] [PubMed]
Cite this article as: Arnold A, Tracy K, Pavkov I, Schlafly M, Holmes K, Chibane F, Kye B, Aiyar A, Pottayil F. Current practices regarding pre-pectoral breast reconstruction following mastectomy for breast cancer: an ASBrS survey gauging support for a national registry. Ann Breast Surg 2025;9:9.

